Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**DOSAGE REGIMEN AND ADMINISTRATION** **Dosage regimen** **Plaque psoriasis** **Adult patients** The recommended dose is 300 mg by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3, and 4 followed by monthly maintenance dosing. Based on clinical response, a maintenance dose of 300 mg every 2 weeks may provide additional benefit for patients with a body weight of 90 kg or higher. Each 300 mg dose is given as one subcutaneous injection of 300mg or as two subcutaneous injections of 150 mg. For some patients, a dosage of 150 mg may be acceptable. **Pediatric patients** The recommended dose is based on body weight (Table 1) and administered by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3, and 4 followed by monthly maintenance dosing (every 4 weeks). Each 75 mg dose is given as one subcutaneous injection of 75 mg. Each 150 mg dose is given as one subcutaneous injection of 150 mg. Each 300 mg dose is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg. 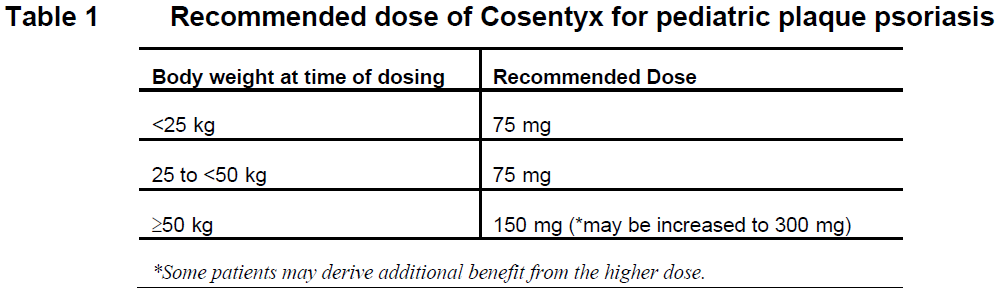 **Psoriatic arthritis** The recommended dose is 150 mg by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3, and 4 followed by monthly maintenance dosing. Based on clinical response, the dose can be increased to 300 mg. For patients with concomitant moderate to severe plaque psoriasis, the dosage and administration for adult plaque psoriasis is recommended. For patients who are anti-TNF-alpha inadequate responders (IR), the recommended dose is 300 mg by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3, and 4 followed by monthly maintenance dosing. Each 300 mg dose is given as one subcutaneous injection of 300mg or as two subcutaneous injections of 150 mg. **Ankylosing spondylitis (AS)** The recommended dose is 150 mg by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3, and 4 followed by monthly maintenance dosing. Based on clinical response, the dose can be increased to 300 mg. Each 300 mg dose is given as one subcutaneous injection of 300mg or as two subcutaneous injections of 150 mg. For all the above indications, available data suggest that a clinical response is usually achieved within 16 weeks of treatment. Consideration should be given to discontinuing treatment in patients who have shown no response by 16 weeks of treatment. Some patients with an initial partial response may subsequently improve with continued treatment beyond 16 weeks. **Non-radiographic axial spondyloarthritis (nr-axSpA)** The recommended dose is 150 mg by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3, and 4 followed by monthly maintenance dosing. **Juvenile Idiopathic Arthritis (JIA)** **Enthesitis-Related Arthritis (ERA) and Juvenile Psoriatic Arthritis (JPsA)** The recommended dose is based on body weight. For patients weighing < 50 kg the dose is 75 mg. For patients weighing ≥ 50 kg the dose is 150 mg. Cosentyx is administered by subcutaneous injection at Weeks 0, 1, 2, 3, and 4 followed by monthly maintenance dosing (every 4 weeks). Each 75 mg dose is given as one subcutaneous injection of 75 mg. Each 150 mg dose is given as one subcutaneous injection of 150 mg. COSENTYX may be administered with or without methotrexate. **Hidradenitis Suppurativa** The recommended dose is 300 mg of secukinumab by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. Based on clinical response, the maintenance dose can be increased to 300 mg every 2 weeks. Each 300 mg dose is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg. **Special populations** **Renal impairment / hepatic impairment** Cosentyx has not been studied specifically in these patient populations. **Pediatric patients** Limited data in pediatric patients with the JIA categories of ERA and JPsA below the age of 6 years. Safety and effectiveness of pediatric patients with plaque psoriasis below the age of 6 years have not been established. Safety and effectiveness in pediatric patients below the age of 18 years in other indications have not yet been established. **Geriatric patients (65 years or above)** No dose adjustment is required. **Method of administration** **Pre-filled syringe & pre-filled pen** Cosentyx is administered by subcutaneous injection. If possible, areas of the skin that show psoriasis should be avoided as injection sites. After proper training in subcutaneous injection technique, patients may self-inject Cosentyx or be injected by a caregiver if a physician determines that it is appropriate. However, the physician should ensure appropriate follow-up of patients. Patients and/or caregivers should be instructed to inject the full amount of Cosentyx according to the instructions provided in the package leaflet. Comprehensive instructions for administration are given in the package leaflet – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. For patients receiving the 75 mg dose, the 75 mg/0.5 mL pre-filled syringe should be used. Full instructions for use are provided in section PHARMACEUTICAL INFORMATION – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
SUBCUTANEOUS
Medical Information
**INDICATIONS** **Plaque psoriasis** Cosentyx is indicated for the treatment of moderate to severe plaque psoriasis in patients 6 years and older who are candidates for systemic therapy or phototherapy (See section CLINICAL STUDIES – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) **Psoriatic arthritis** Cosentyx is indicated for the treatment of active psoriatic arthritis in adult patients when the response to previous disease-modifying anti-rheumatic drug (DMARD) therapy has been inadequate. Cosentyx can be used alone or in combination with methotrexate. **Axial spondyloarthritis (axSpA) with or without radiographic damage** **Ankylosing spondylitis (AS)/ axSpA with radiographic damage** Cosentyx is indicated for the treatment of active ankylosing spondylitis in adult patients who have responded inadequately to conventional therapy. **Non-radiographic axial spondyloarthritis (nr-axSpA) / axSpA without radiographic damage** Cosentyx is indicated for the treatment of adult patients with active non-radiographic axial spondyloarthritis with objective signs of inflammation, as indicated by elevated C-reactive protein (CRP) and/or magnetic resonance imaging (MRI) evidence who have had an inadequate response to, or are intolerant to nonsteroidal anti-inflammatory drugs (NSAIDs). **Juvenile Idiopathic Arthritis (JIA)** **Enthesitis-Related Arthritis (ERA)** Cosentyx is indicated for the treatment of active enthesitis-related arthritis in patients 6 years and older whose disease has responded inadequately to, or who cannot tolerate, conventional therapy (see CLINICAL TRIAL section – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Juvenile Psoriatic Arthritis (JPsA)** Cosentyx is indicated for the treatment of active juvenile psoriatic arthritis in patients 6 years and older whose disease has responded inadequately to, or who cannot tolerate, conventional therapy (see CLINICAL TRIAL section – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hidradenitis Suppurativa (HS)** Cosentyx is indicated for the treatment of moderate to severe hidradenitis suppurativa (acne inversa) in adult patients with an inadequate response to conventional systemic therapy (See clinical trials section – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**CONTRAINDICATIONS** Severe hypersensitivity reactions to the active substance or to any of the excipients (see sections DESCRIPTION AND COMPOSITION, WARNINGS AND PRECAUTIONS and ADVERSE DRUG REACTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
L04AC10
secukinumab
Manufacturer Information
NOVARTIS (SINGAPORE) PTE LTD
Novartis Pharma Stein AG
Active Ingredients
Documents
Package Inserts
Cosentyx Solution For Injection_PI.pdf
Approved: May 11, 2023
