Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Posology and method of administration** Epclusa treatment should be initiated and monitored by a physician experienced in the management of patients with HCV infection. Posology The recommended dose of Epclusa in adults is one 400 mg/100 mg tablet, taken orally, once daily with or without food as detailed in Table 1 (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The recommended dose of Epclusa in patients aged 12 to < 18 years and weighing at least 30 kg is one 400 mg/100 mg tablet, taken orally, once daily with or without food for 12 weeks (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 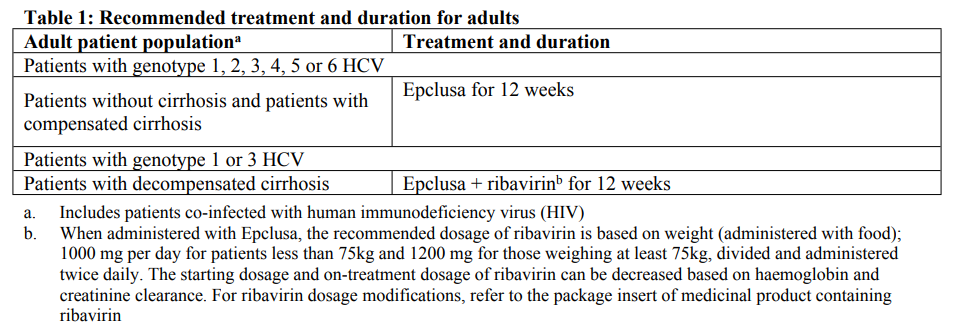 Patients should be instructed that if vomiting occurs within 3 hours of dosing an additional tablet of Epclusa should be taken. If vomiting occurs more than 3 hours after dosing, no further dose of Epclusa is needed (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If a dose of Epclusa is missed and it is within 18 hours of the normal time, patients should be instructed to take the tablet as soon as possible and then patients should take the next dose at the usual time. If it is after 18 hours then patients should be instructed to wait and take the next dose of Epclusa at the usual time. Patients should be instructed not to take a double dose of Epclusa. _Elderly_ No dose adjustment is warranted for elderly patients (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment_ No dose adjustment of Epclusa is required for patients with mild or moderate renal impairment. Safety data are limited in patients with severe renal impairment (estimated glomerular filtration rate \[eGFR\] < 30 mL/min/1.73 m2) and end stage renal disease (ESRD) requiring haemodialysis. Epclusa can be used in these patients with no dose adjustment (see section 4.4, 4.8, 5.1 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Hepatic impairment_ No dose adjustment of Epclusa is required for patients with mild, moderate, or severe hepatic impairment (CPT Class A, B, or C) (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Safety and efficacy of Epclusa have been assessed in adult patients with CPT Class B cirrhosis, but not in patients with CPT Class C cirrhosis (see sections 4.4, 4.8 and 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Paediatric population_ The safety and efficacy of Epclusa in children aged less than 12 years or weighing less than 30 kg have not yet been established. Method of administration For oral use. Patients should be instructed to swallow the tablet whole with or without food (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Due to the bitter taste, it is recommended that the film-coated tablet is not chewed or crushed.
ORAL
Medical Information
**4.1 Therapeutic indications** Epclusa is indicated for the treatment of chronic hepatitis C virus (HCV) infection in patients aged 12 years and older and weighing at least 30kg (see sections 4.2, 4.4 and 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**4.3 Contraindications** Hypersensitivity to the active substances or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Use with strong P-gp and strong CYP inducers Medicinal products that are strong P-glycoprotein (P-gp) and/or strong cytochrome P450 (CYP) inducers (carbamazepine, phenobarbital, phenytoin, rifampicin, rifabutin and St. John’s wort). Co-administration will significantly decrease sofosbuvir or velpatasvir plasma concentrations and could result in loss of efficacy of Epclusa (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
J05AP55
sofosbuvir and velpatasvir
Manufacturer Information
GILEAD SCIENCES SINGAPORE PTE. LTD.
Hovione FarmaCiencia, S.A. (Drug product intermediate)
Hovione Limited (Drug product intermediate)
Patheon Inc.
Gilead Sciences Ireland UC
Esteve Quimica S.A. (Drug product intermediate)
Active Ingredients
Documents
Package Inserts
Epclusa Tablet PI.pdf
Approved: December 21, 2021
