Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**DOSAGE AND ADMINISTRATION** General Dosing Guidelines: Prescribers should consult the full prescribing information and clinical study information of protease inhibitors if they are co-administered with a reduced dose of ritonavir. _**Adults**_ _**Tablets**_ The recommended dose of ritonavir tablets is 600 mg (six tablets) twice daily by mouth and should be given with food. Ritonavir tablets should be swallowed whole and not chewed, broken or crushed. Patients who take the 600 mg twice daily soft gel capsule dose may experience more gastrointestinal side effects such as nausea, vomiting, abdominal pain or diarrhea when switching from the soft gel capsule to the tablet formulation because of greater maximum plasma concentration (Cmax) achieved with the tablet formulation relative to the soft gel capsule (see **CLINICAL PHARMACOLOGY** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Use of a dose titration schedule may help to reduce treatment emergent adverse events while maintaining appropriate ritonavir plasma levels. Ritonavir should be started at no less than 300 mg twice daily for a period of three days and increased by 100 mg twice daily increments up to 600 mg twice daily over a period of no longer than 14 days. Patients should be aware that frequently observed adverse events, such as mild to moderate gastrointestinal disturbances and paresthesias, may diminish as therapy is continued. Patients should not remain on 300 mg twice daily for more than three days. **_Pediatric Patients_** Ritonavir should be used in combination with other antiretroviral agents. The recommended dosage of ritonavir is 400 mg/ m2 of body surface area twice daily by mouth and should not exceed 600 mg twice daily. Ritonavir should be started at 250 mg/m2 and increased at two to three day intervals by 50 mg/ m2 twice daily until the recommended twice daily dose is achieved. If patients do not tolerate the maximum daily dose due to adverse events, the highest tolerated dose should be used for maintenance therapy in combination with other antiretroviral agents. 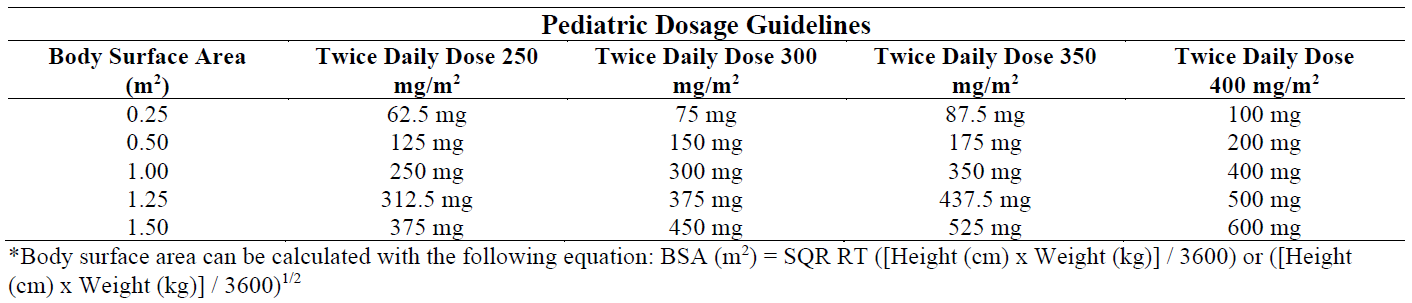 The tablet formulation may not be suitable for paediatric use in some cases.
ORAL
Medical Information
**INDICATIONS AND USAGE** Ritonavir is indicated in combination with other antiretroviral agents for the treatment of patients with HIV-1 infection when therapy is warranted based on clinical and/or immunological evidence of disease progression.
**CONTRAINDICATIONS** Ritonavir is contraindicated in patients with known hypersensitivity to ritonavir or any of its formulation excipients. When co-administering ritonavir with other protease inhibitors, see the full prescribing information for that protease inhibitor including contraindication information. In vitro studies have demonstrated that ritonavir is a potent inhibitor of many cytochrome P450 mediated biotransformations. Based primarily on literature review, ritonavir is expected to produce large increases in the plasma concentrations of the drugs metabolized by cytochrome P450. Co-administration of ritonavir is contraindicated with the drugs listed in Table 1. 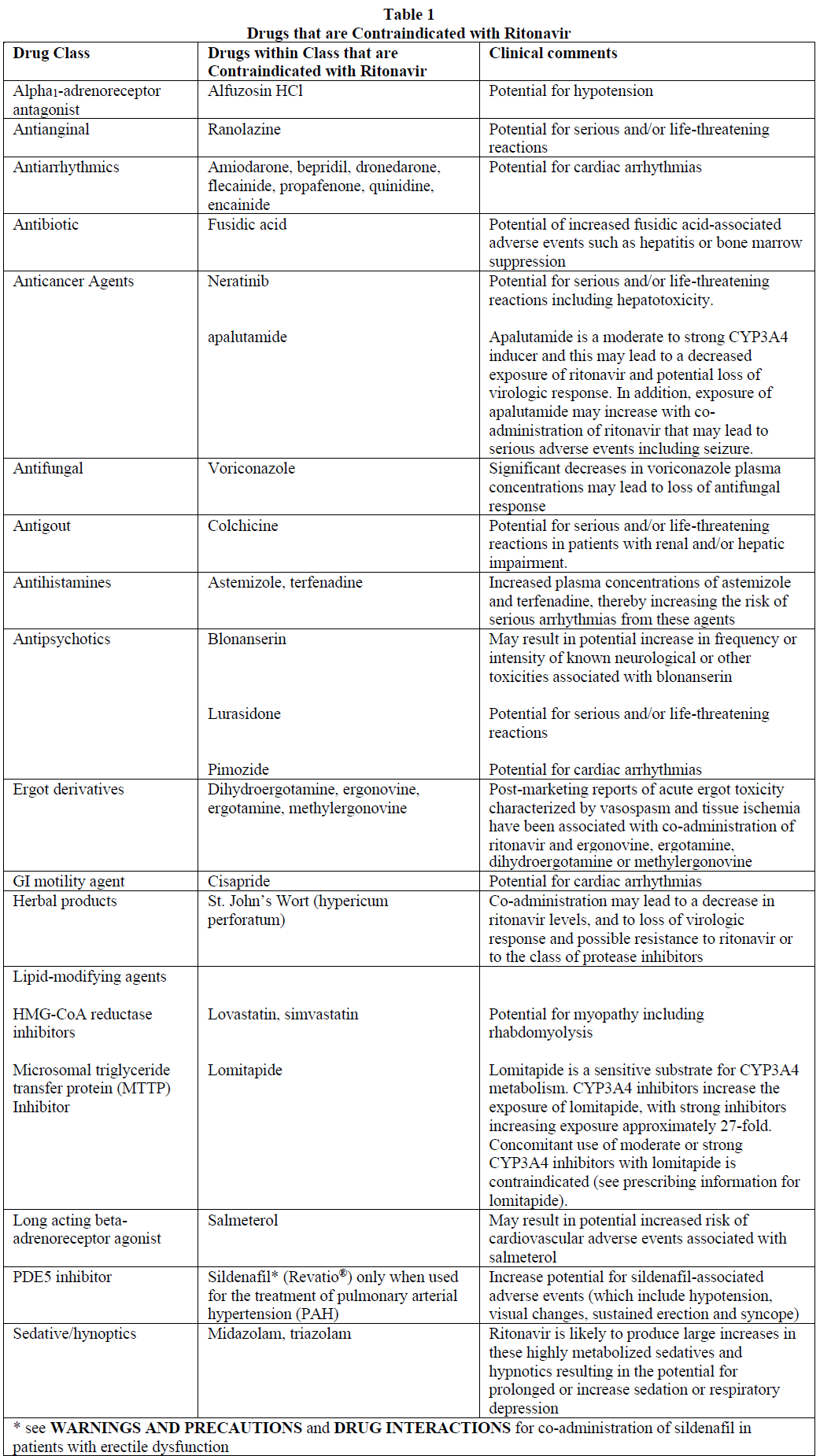
J05AE03
ritonavir
Manufacturer Information
ABBVIE PTE. LTD.
AbbVie Deutschland GmbH & Co. KG
Active Ingredients
Documents
Package Inserts
NORVIR TABLETS PI.pdf
Approved: January 12, 2023
