Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**4.2 Posology and method of administration** Treatment must be initiated and monitored under the supervision of physicians experienced in the management of multiple myeloma. Dosing is continued or modified based upon clinical and laboratory findings (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Posology - _Pomalidomide in combination with bortezomib and dexamethasone_ The recommended starting dose of Pomalyst® is 4 mg orally once daily on Days 1 to 14 of repeated 21-day cycles. Pomalidomide is administered in combination with bortezomib and dexamethasone, as shown in Table 1. The recommended starting dose of bortezomib is 1.3 mg/m2 intravenous or subcutaneous once daily, on the days shown in Table 1. The recommended dose of dexamethasone is 20 mg orally once daily, on the days shown in Table 1. Treatment with pomalidomide combined with bortezomib and dexamethasone should be given until disease progression or until unacceptable toxicity occurs. 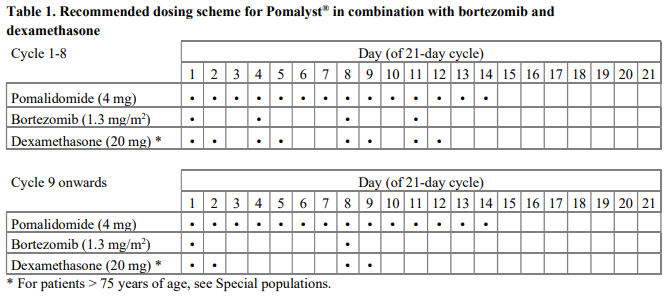 _Pomalidomide dose modification or interruption_ To initiate a new cycle of pomalidomide, the neutrophil count must be ≥ 1 x 109/l and the platelet count must be ≥ 50 x 109/l. Instructions on dose interruptions or reductions for pomalidomide-related adverse reactions are outlined in the Table 2 and dose levels are defined in Table 3 below: 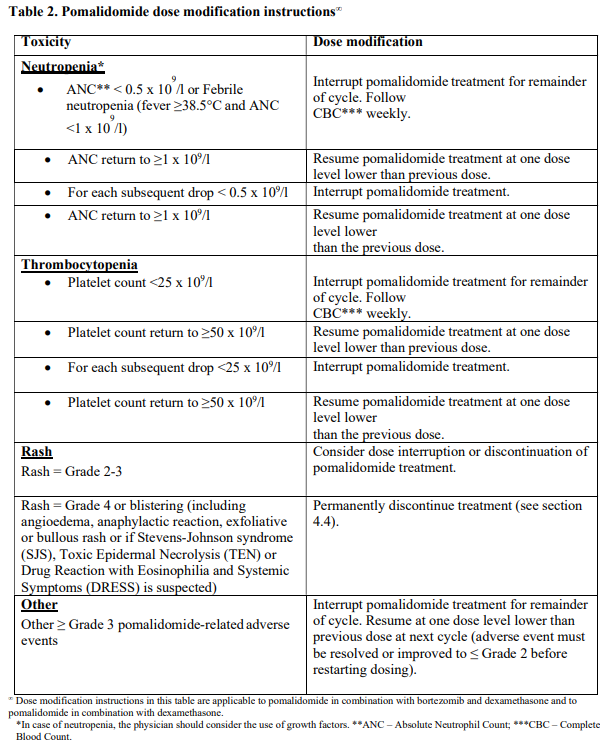 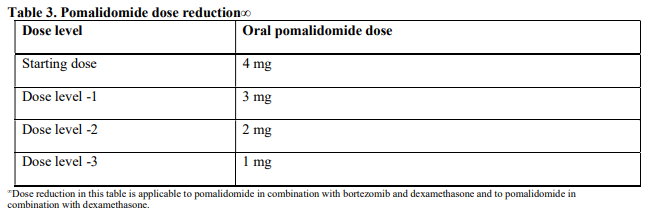 If adverse reactions occur after dose reductions to 1 mg, then the medicinal product should be discontinued. Strong CYP1A2 inhibitors If strong inhibitors of CYP1A2 (e.g. ciprofloxacin, enoxacin and fluvoxamine) are co-administered with pomalidomide, reduce the dose of pomalidomide by 50% (see sections 4.5 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Bortezomib dose modification or interruption_ For instructions on dose interruptions or reductions for bortezomib-related adverse reactions, physicians should refer to bortezomib Package Insert. _Dexamethasone dose modification or interruption_ Instructions on dose interruptions or reductions for low-dose dexamethasone-related adverse reactions are outlined in Tables 4 and 5 below. However, dose interruption or resumption decisions are at the physician’s discretion per Package Insert. 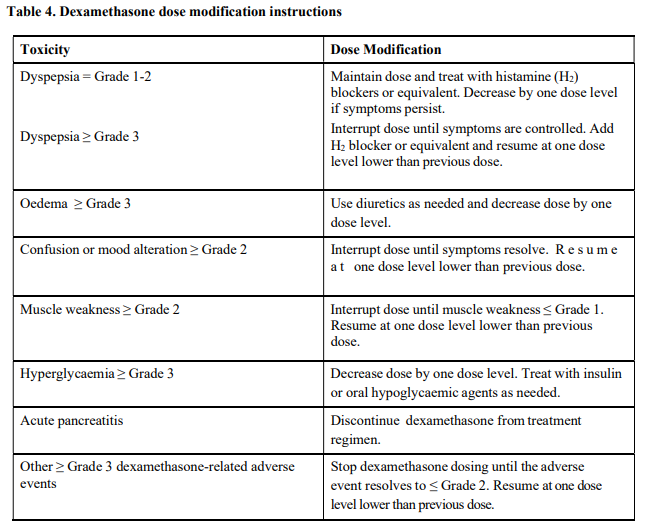 If recovery from toxicities is prolonged beyond 14 days, then the dose of dexamethasone will be resumed at one dose level lower than the previous dose. 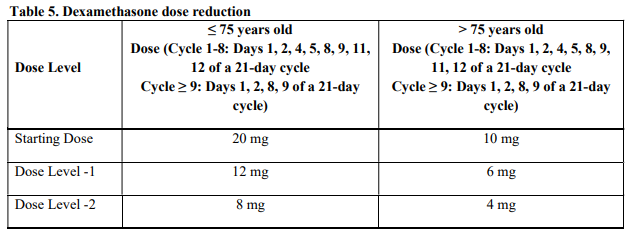 Dexamethasone should be discontinued if the patient is unable to tolerate 8 mg if ≤ 75 years old or 4 mg if > 75 years old. In case of permanent discontinuation of any component of the treatment regimen, continuation of the remaining medicinal products is at the physician’s discretion. - _Pomalidomide in combination with dexamethasone_ The recommended starting dose of Pomalyst® is 4 mg orally once daily on Days 1 to 21 of each 28-day cycle. The recommended dose of dexamethasone is 40 mg orally once daily on Days 1, 8, 15 and 22 of each 28-day cycle. Treatment with pomalidomide combined with dexamethasone should be given until disease progression or until unacceptable toxicity occurs. _Pomalidomide dose modification or interruption_ Instructions for dose interruptions or reductions for pomalidomide-related adverse reactions are outlined in Table 2 and 3. _Dexamethasone dose modification or interruption_ Instructions for dose modification for dexamethasone-related adverse reactions are outlined in Table 4. Instructions for dose reduction for dexamethasone-related adverse reactions are outlined in Table 6 below. However, dose interruption / resumption decisions are at physician’s discretion per the current Package Insert. 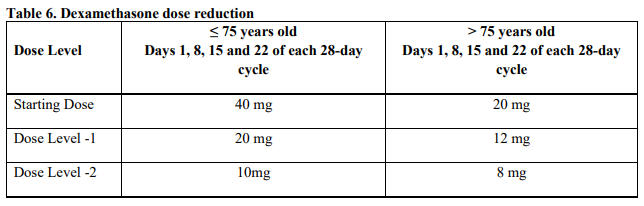 Dexamethasone should be discontinued if the patient is unable to tolerate 10 mg if ≤ 75 years old or 8 mg if > 75 years old. Special populations _Elderly_ - _Pomalidomide in combination with bortezomib and dexamethasone_ No dose adjustment is required for pomalidomide. For information on bortezomib given in combination with Pomalyst®, refer to the respective current Package Insert. For patients >75 years of age, the starting dose of dexamethasone is: - For Cycles 1 to 8: 10 mg once daily on Days 1, 2, 4, 5, 8, 9, 11 and 12 of each 21-day cycle - For Cycles 9 and onwards: 10 mg once daily on Days 1, 2, 8 and 9 of each 21-day cycle. - _Pomalidomide in combination with dexamethasone_ No dose adjustment is required for pomalidomide. For patients > 75 years of age, the starting dose of dexamethasone is: - 20 mg once daily on days 1, 8, 15 and 22 of each 28-day cycle. _Renal impairment_ No dose adjustment of pomalidomide is required for patients with renal impairment. On haemodialysis days, patients should take their pomalidomide dose following haemodialysis. _Hepatic impairment_ Patients with serum total bilirubin > 1.5 x ULN (upper limit of normal range) were excluded from clinical studies. Hepatic impairment has a modest effect on the pharmacokinetics of pomalidomide (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). No adjustment of the starting dose of pomalidomide is required for patients with hepatic impairment as defined by the Child-Pugh criteria. However, patients with hepatic impairment should be carefully monitored for adverse reactions and dose reduction or interruption of pomalidomide should be used as needed. _Paediatric population_ There is no relevant use of Pomalyst® in children aged 0–17 years for the indication of multiple myeloma. Method of administration Oral use. Pomalyst® should be taken orally at the same time each day. The capsules should not be opened, broken or chewed (see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). This medicinal product should be swallowed whole, preferably with water, with or without food. If the patient forgets to take a dose of Pomalyst® on one day, then the patient should take the normal prescribed dose as scheduled on the next day. Patients should not adjust the dose to make up for a missing dose on previous days. It is recommended to press only on one end of the capsule to remove it from the blister thereby reducing the risk of capsule deformation or breakage. For information on other medicinal products given in combination with Pomalyst®, refer to the respective current Package Insert.
ORAL
Medical Information
**4.1 Therapeutic indications** Pomalyst® in combination with bortezomib and dexamethasone is indicated in the treatment of adult patients with multiple myeloma who have received at least one prior treatment regimen including lenalidomide. Pomalyst® in combination with dexamethasone is indicated in the treatment of adult patients with relapsed and refractory multiple myeloma who have received at least two prior treatment regimens, including both lenalidomide and bortezomib, and have demonstrated disease progression on the last therapy.
**4.3 Contraindications** - Pregnancy. - Women of childbearing potential, unless all the conditions of the pregnancy prevention programme are met (see sections 4.4 and 4.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Male patients unable to follow or comply with the required contraceptive measures (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. For information on other medicinal products given in combination with Pomalyst®, refer to the respective current Package Insert.
L04AX06
pomalidomide
Manufacturer Information
CELGENE PTE. LTD.
Celgene International Sarl
