Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**2 DOSAGE AND ADMINISTRATION** **2.1 General** Daptomycin for injection is given by intravenous (IV) administration. Daptomycin for injection is a sterile product contained in a single-dose vial. **2.2 Adults** **Complicated Skin and Skin Structure Infections** Daptomycin for injection 4 mg/kg is administered to adult patients intravenously in 0.9% sodium chloride for injection once every 24 hours for 7 to 14 days, either by injection over a 2-minute period or by infusion over a 30-minute period. Do not dose Daptomycin for injection more frequently than once a day, and measure creatine phosphokinase (CPK) levels at baseline and at regular intervals (at least weekly). _\[See 3 INSTRUCTIONS FOR USE, 3.1 Preparation of Daptomycin for injection for Administration_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _.\]_ **_Staphylococcus aureus_ Bloodstream Infections (Bacteremia)** Daptomycin for injection 6 mg/kg is administered to adult patients intravenously in 0.9% sodium chloride for injection once every 24 hours for 2 to 6 weeks, either by injection over a 2-minute period or by infusion over a 30-minute period. Duration of treatment is based on the treating physician's working diagnosis. Do not dose Daptomycin for injection more frequently than once a day, and measure CPK levels at baseline and at regular intervals (at least weekly). _\[See 3 INSTRUCTIONS FOR USE, 3.1 Preparation of Daptomycin for injection for Administration_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _.\]_ **2.3 Pediatric Patients (1 to 17 Years of Age)** **Complicated Skin and Skin Structure Infections** The recommended dosage regimens based on age for pediatric patients with cSSSI are shown in Table 1. Daptomycin for injection should be administered intravenously in 0.9% sodium chloride for injection once every 24 hours for up to 14 days. **Unlike in adults, Daptomycin for injection should not be administered by injection over a two (2) minute period in pediatric patients.** 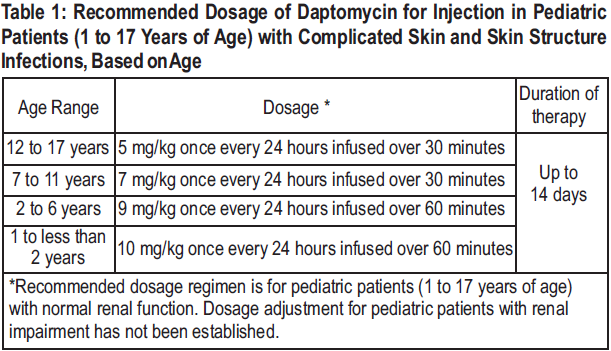 **_Staphylococcus aureus_ Bloodstream Infections (Bacteremia)** The recommended dosage regimens based on age for pediatric patients with _S. aureus_ bloodstream infections (bacteremia) are shown in Table 2. Daptomycin for injection should be administered intravenously in 0.9% sodium chloride for injection once every 24 hours for up to 42 days. 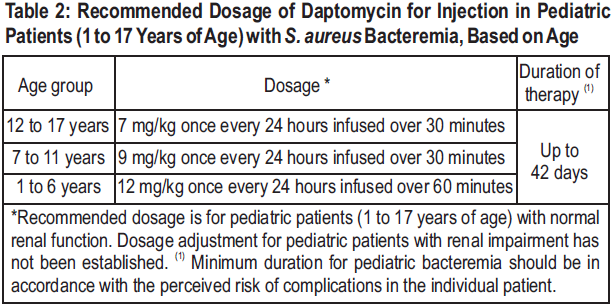 **2.4 Renal Impairment** Daptomycin is eliminated primarily by the kidneys; therefore, an adjustment of Daptomycin for injection dosage interval is recommended for adult patients with creatinine clearance (CLCR) <30 mL/min, including patients receiving hemodialysis or continuous ambulatory peritoneal dialysis (CAPD). The recommended dosing regimen for these adult patients is 4 mg/kg (cSSSI) or 6 mg/kg ( _S. aureus_ bloodstream infections) once every 48 hours. When possible, administer Daptomycin for injection following the completion of hemodialysis on hemodialysis days. In adult patients with renal impairment, monitor both renal function and CPK more frequently than once weekly. No dosage interval adjustment is required for adult patients with CLCR ≥30 mL/min. Due to limited clinical experience, Daptomycin for injection should only be used in adult patients with any degree of renal impairment (creatinine clearance <80 mL/min) when it is considered that the expected clinical benefit outweighs the potential risk. The response to treatment and renal function should be closely monitored in all adult patients with some degree of renal impairment. 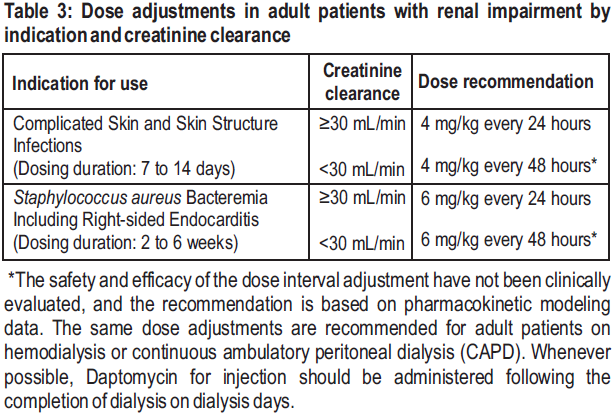 The dosage regimen for Daptomycin for injection in pediatric patients with renal impairment has not been established.
INTRAVENOUS
Medical Information
**1 INDICATIONS AND USAGE** **1.1 Complicated Skin and Skin Structure Infections (cSSSI)** Adult (≥18 years of age) and pediatric (1 to 17 years of age) patients with complicated skin and skin structure infections (cSSSI) caused by susceptible isolates of the following Gram-positive bacteria: _Staphylococcus aureus_ (including methicillin-resistant isolates), _Streptococcus pyogenes_, _Streptococcus agalactiae_, _Streptococcus dysgalactiae_ subsp. _equisimilis_, and _Enterococcus faecalis_ (vancomycin-susceptible isolates only). **1.2 _Staphylococcus aureus_ Bloodstream Infections (Bacteremia)** Adult patients (≥18 years of age) with _Staphylococcus aureus_ bloodstream infections (bacteremia), including those with right-sided infective endocarditis (SAB/RIE), caused by methicillin-susceptible and methicillin-resistant isolates. Pediatric patients (1 to 17 years of age) with _S. aureus_ bloodstream infections (bacteremia) caused by methicillin-susceptible and methicillin-resistant isolates. Daptomycin for injection is not indicated for the treatment of left-sided infective endocarditis due to _S. aureus_. The efficacy of daptomycin for injection in patients with left-sided infective endocarditis due to _S. aureus_ has not been demonstrated. The clinical trial of Daptomycin for injection in patients with _S.aureus_ bloodstream infections included limited data from patients with left-sided infective endocarditis; outcomes in these patients were poor. Daptomycin for injection has not been studied in patients with prosthetic valve endocarditis. Daptomycin for injection is not indicated for the treatment of pneumonia _\[See 5 WARNINGS AND PRECAUTIONS, 5.2 Pneumonia_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_.
**4 CONTRAINDICATIONS** Daptomycin for injection is contraindicated in patients with known hypersensitivity to daptomycin.
J01XX09
daptomycin
Manufacturer Information
WELLESTA HEALTHCARE (SINGAPORE) PTE. LTD.
Biological E. Limited
Active Ingredients
Documents
Package Inserts
BEDAPT DAPTOMYCIN POWDER FOR SOLUTION FOR INJECTION OR INFUSION PI.pdf
Approved: July 31, 2023
