Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**4.2 Posology and method of administration** Treatment with REKOVELLE should be initiated under the supervision of a physician experienced in the treatment of fertility problems. Patients must be educated on how to use the REKOVELLE injection pen and to perform injections. Posology The posology of REKOVELLE is individualised for each patient to obtain an ovarian response with a favourable safety/efficacy profile (see Section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). REKOVELLE is dosed in micrograms (μg) and not in international units (IU) of biological activity (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The dosing regimen is specific for REKOVELLE and the microgram dose cannot be applied to other gonadotropins. For the first treatment cycle, the individual daily dose will be determined on the basis of the woman’s serum anti-Müllerian hormone (AMH) concentration, which is a biomarker of ovarian response to gonadotropins, and her body weight. The dose should be based on a recent determination of AMH (i.e. within the last 12 months) measured by the following diagnostic tests: Elecsys® AMH Plus immunoassay from Roche (i.e. assay used in clinical development trials). The individual daily dose is to be maintained throughout the stimulation period. For women with AMH <15 pmol/L the daily dose is 12 micrograms, irrespective of body weight. For women with AMH ≥15 pmol/L the daily dose decreases from 0.19 to 0.10 micrograms/kg by increasing AMH concentration (Table 1). The dose is to be rounded off to the nearest 0.33 micrograms to match the dosing scale on the injection pen. The maximum daily dose for the first treatment cycle is 12 micrograms. The AMH concentration is to be expressed in pmol/L and is to be rounded off to the nearest integer (Table 1). If the AMH concentration is in ng/mL, the concentration should be converted to pmol/L by multiplying with 7.14 (ng/mL x 7.14 = pmol/L) before use. For calculation of the REKOVELLE dose, the body weight is to be measured without shoes and overcoat just prior to start of stimulation. 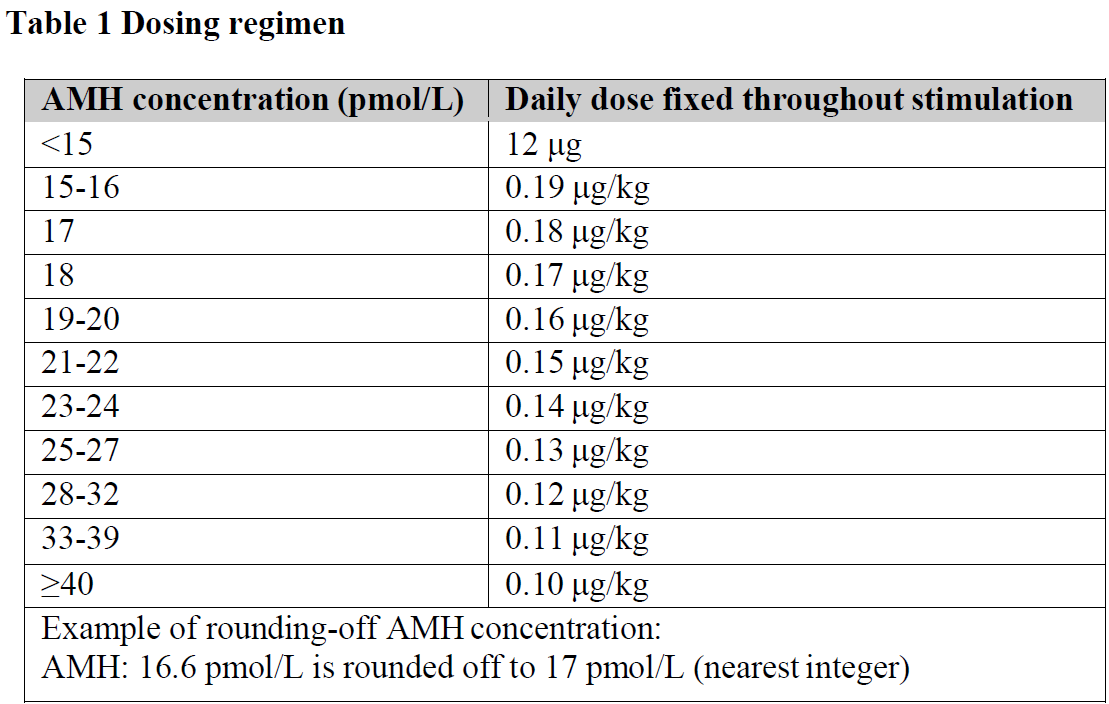 Dosing with REKOVELLE should be initiated day 2 or 3 after start of menstrual bleeding, and continue until adequate follicular development has been achieved as assessed by monitoring with ultrasound alone or in combination with measurement of serum estradiol levels. Adequate follicular development is achieved on average by the ninth day of treatment (range 5 to 20 days). As soon as ≥3 follicles ≥17 mm are observed, a single injection of 250 micrograms recombinant human chorionic gonadotropin (hCG) or 5,000 international units hCG is administered to induce final follicular maturation. In patients with excessive ovarian response at risk of ovarian hyperstimulation syndrome (OHSS), administration of a GnRH agonist instead of hCG could be considered for triggering of final follicular maturation. Administration of GnRH agonist can reduce, but not eliminate, the risk for OHSS and is applicable only for GnRH antagonist cycles. In case of GnRH agonist administration, embryos should not be replaced in the fresh cycle but cryopreserved for later use. In patients with excessive ovarian response of >35 follicles with a diameter ≥12 mm, triggering of final follicular maturation should not be performed and the cycle cancelled. For subsequent treatment cycles, the daily dose of REKOVELLE should be maintained or modified according to the patient’s ovarian response in the previous cycle. If the patient had adequate ovarian response in the previous cycle without developing OHSS, the same daily dose of REKOVELLE should be used. In case of ovarian hypo-response in the previous cycle, the daily dose of REKOVELLE in the subsequent cycle should be increased by 25% or 50%, according to the extent of response observed. In case of ovarian hyper-response in the previous cycle, the daily dose of REKOVELLE in the subsequent cycle should be decreased by 20% or 33%, according to the extent of response observed. In patients who developed OHSS or were at risk of OHSS in a previous cycle, the daily dose of REKOVELLE for the subsequent cycle is 33% lower than the dose used in the cycle where OHSS or risk of OHSS occurred. The maximum daily dose is 24 micrograms. There is no clinical trial experience with REKOVELLE in the long GnRH agonist protocol. Elderly (more than 65 years) There is no relevant use of REKOVELLE in the elderly population. Safety and efficacy of REKOVELLE in elderly patients have not been established. Patients with renal and hepatic impairment Safety, efficacy and pharmacokinetics of REKOVELLE in patients with renal or hepatic impairment have not been established. Polycystic ovarian syndrome patients with anovulatory disorders Polycystic ovarian syndrome patients with anovulatory disorders have not been studied. Paediatric population There is no relevant use of REKOVELLE in the paediatric population for the indication. Method of administration REKOVELLE is intended for subcutaneous administration, preferably in the abdominal wall. The first injection of REKOVELLE should be performed under direct medical supervision. Self-administration of REKOVELLE should only be performed by patients who are well motivated, adequately trained and have access to expert advice. For instructions on the administration with the pre-filled pen, see the “Instructions for Use” – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
SUBCUTANEOUS
Medical Information
**4.1 Therapeutic indications** Controlled ovarian stimulation for the development of multiple follicles in women undergoing assisted reproductive technologies (ART) such as an _in vitro_ fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) cycle.
**4.3 Contraindications** - Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ - Tumours of the hypothalamus or pituitary gland - Ovarian enlargement or ovarian cyst not due to polycystic ovarian syndrome - Gynaecological haemorrhages of unknown aetiology - Ovarian, uterine or mammary carcinoma - Pregnancy and lactation REKOVELLE must not be used when an effective response cannot be obtained, such as: - Primary ovarian failure - Malformations of sexual organs incompatible with pregnancy - Fibroid tumours of the uterus incompatible with pregnancy
G03GA10
follitropin delta
Manufacturer Information
FERRING PHARMACEUTICALS PRIVATE LIMITED
Vetter Pharma-Fertigung GmbH & Co. KG (Bulk Production and Primary Packager)
Active Ingredients
Documents
Package Inserts
Rekovelle Injection PI.pdf
Approved: August 25, 2021
