Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 POSOLOGY AND METHOD OF ADMINISTRATION** For oral use once daily with or without food **Adjunctive treatment of Major Depressive Disorder (Adults)** The recommended starting dose for brexpiprazole as adjunctive treatment of MDD in adults is 0.5 mg or 1 mg once daily. Dose titration to 1 mg/day and up to the target dose of 2 mg/day should occur at intervals of up to 1 week based on the patient’s clinical response and tolerability. Doses up to 3 mg/day have been studied in clinical trials. The benefit of the 3 mg dose has not been clearly established (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Periodically reassess to determine the continued need and appropriate dose for treatment. The long-term efficacy of brexpiprazole as adjunctive treatment in MDD has not been established. **Schizophrenia (Adults and Pediatric Patients 13 to 17 Years)** **Adults** The recommended starting dose for brexpiprazole in the treatment of adult patients with schizophrenia is 1 mg once daily on days 1 to 4. The recommended target dose range is 2 mg to 4 mg once daily. Titrate to 2 mg once daily on Day 5 through Day 7, then to 4 mg on Day 8 based on the patient’s clinical response and tolerability. The maximum recommended daily dosage is 4 mg. _Maintenance treatment:_ The recommended maintenance dose range is 2 mg/day to 4 mg/day. Periodically reassess to determine the continued need for maintenance treatment. **Pediatric Patients (13 to 17 years of age)** The recommended starting dosage for REXULTI for the treatment of schizophrenia in pediatric patients 13 to 17 years of age is 0.5 mg once daily on Days 1 to 4, taken orally with or without food _\[see Special Population (5.2)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Titrate to 1 mg once daily on Day 5 through Day 7, then to 2 mg on Day 8 based on the patient’s clinical response and tolerability. Weekly dose increases can be made in 1 mg increments. The recommended target REXULTI dosage is 2 mg to 4 mg once daily. The maximum recommended daily dosage is 4 mg. **Dosing Adjustment for Hepatic Impairment** For patients with moderate to severe hepatic impairment (Child-Pugh score ≥7), the maximum recommended dosage is 2 mg once daily for patients with MDD, and 3 mg once daily for patients with schizophrenia. **Dosing Adjustment for Renal Impairment** For patients with moderate, severe or end-stage renal impairment (creatinine clearance CrCl<60 mL/minute), the maximum recommended dosage is 2 mg once daily for patients with MDD and 3 mg once daily for patients with schizophrenia. 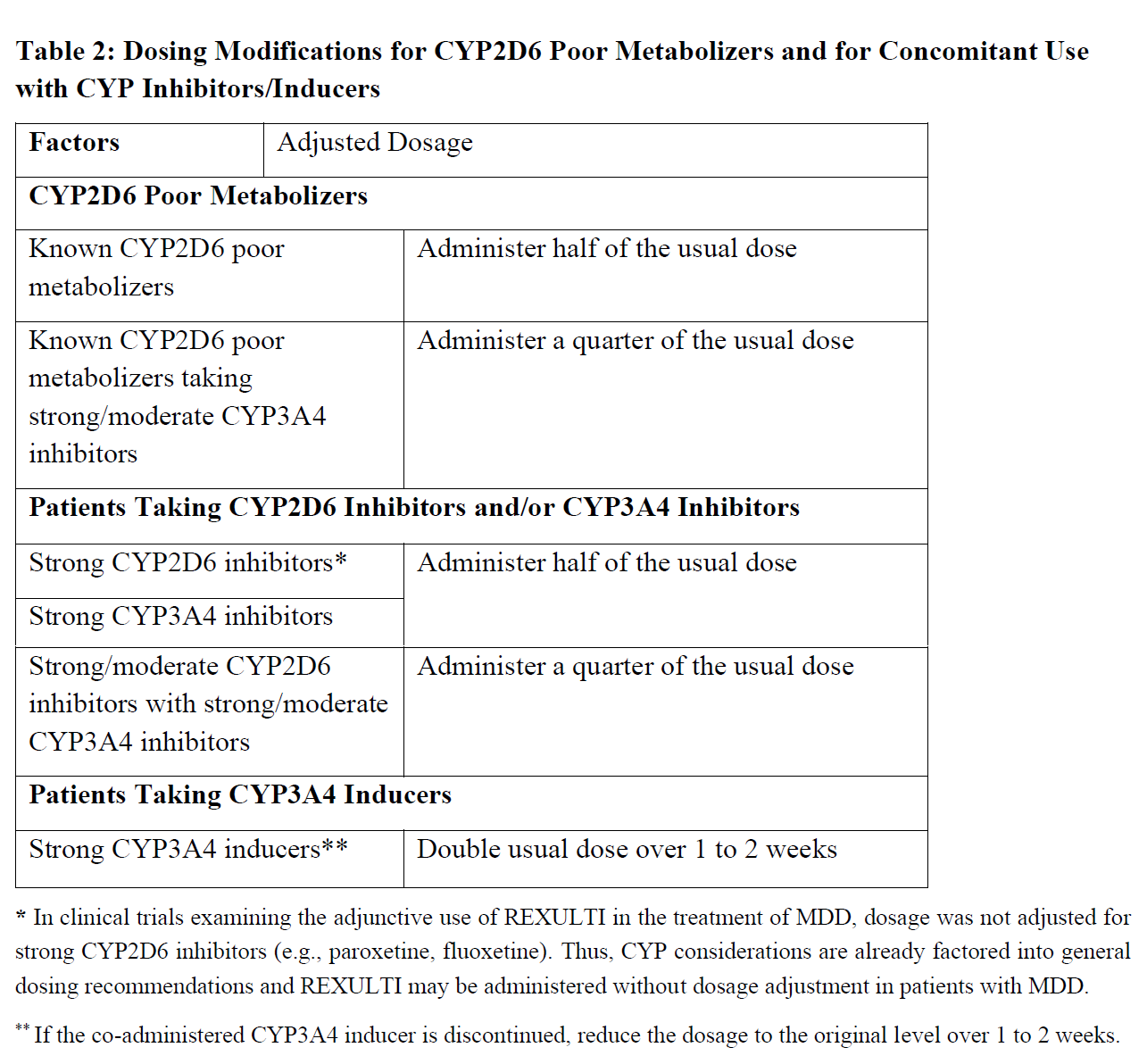
ORAL
Medical Information
**4.1 THERAPEUTIC INDICATIONS** Brexpiprazole is indicated in adult patients for: - Use as an adjunctive therapy to antidepressants for the treatment of major depressive disorder (MDD). Efficacy was demonstrated in 6-week trials in adults with MDD who had an inadequate response to antidepressant therapy during the current episode (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The long-term efficacy of brexpiprazole as adjunctive treatment in MDD has not been established. - Treatment of schizophrenia (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) in adults and pediatric patients aged 13 years and older.
**4.3 CONTRAINDICATIONS** Hypersensitivity to the active substance or to any of the excipients.
N05AX16
brexpiprazole
Manufacturer Information
LUNDBECK SINGAPORE PTE. LTD.
OTSUKA PHARMACEUTICAL CO., LTD. (TOKUSHIMA FACTORY)
ELAIAPHARM (PRIMARY AND SECONDARY PACKAGER)
OTSUKA PHARMACEUTICAL CO., LTD. (SECOND TOKUSHIMA FACTORY) (Finished Product Manufacturer)
Active Ingredients
Documents
Package Inserts
Rexulti Approved PI.pdf
Approved: May 2, 2023
