Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
POWDER, FOR SOLUTION
**4.2. Posology and method of administration** For patients with circulating lymphoblasts, cytoreduction with a combination of hydroxyurea, steroids, and/or vincristine to a peripheral blast count ≤10,000/mm3 is recommended prior to the first dose. Premedication with a corticosteroid, antipyretic, and antihistamine is recommended prior to dosing (see Section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients should be observed during and for at least 1 hour after the end of the infusion for symptoms of infusion related reactions (see Section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Posology Administer inotuzumab ozogamicin in 3- to 4-week cycles. For patients proceeding to a hematopoietic stem cell transplant (HSCT), the recommended duration of treatment with inotuzumab ozogamicin is 2 cycles. A third cycle should be considered for those patients who do not achieve a complete remission (CR) or a complete remission with incomplete hematologic recovery (CRi) and minimal residual disease (MRD) negativity after 2 cycles (see Section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For patients not proceeding to HSCT, a maximum of 6 cycles may be administered. Any patients who do not achieve a CR or CRi within 3 cycles should discontinue treatment. Table 1 shows the recommended dosing regimens. For the first cycle, the recommended total dose of inotuzumab ozogamicin for all patients is 1.8 mg/m2 per cycle, administered as 3 divided doses on Days 1 (0.8 mg/m2), 8 (0.5 mg/m2), and 15 (0.5 mg/m2). Cycle 1 is 3 weeks in duration, but may be extended to 4 weeks if the patient achieves a CR or CRi, and/or to allow recovery from toxicity. For subsequent cycles, the recommended total dose of inotuzumab ozogamicin is 1.5 mg/m2 per cycle, administered as 3 divided doses on Days 1 (0.5 mg/m2), 8 (0.5 mg/m2), and 15 (0.5 mg/m2) for patients who achieve a CR or CRi or 1.8 mg/m2 per cycle given as 3 divided doses on Days 1 (0.8 mg/m2), 8 (0.5 mg/m2), and 15 (0.5 mg/m2) for patients who do not achieve a CR or CRi. Subsequent cycles are 4 weeks in duration. 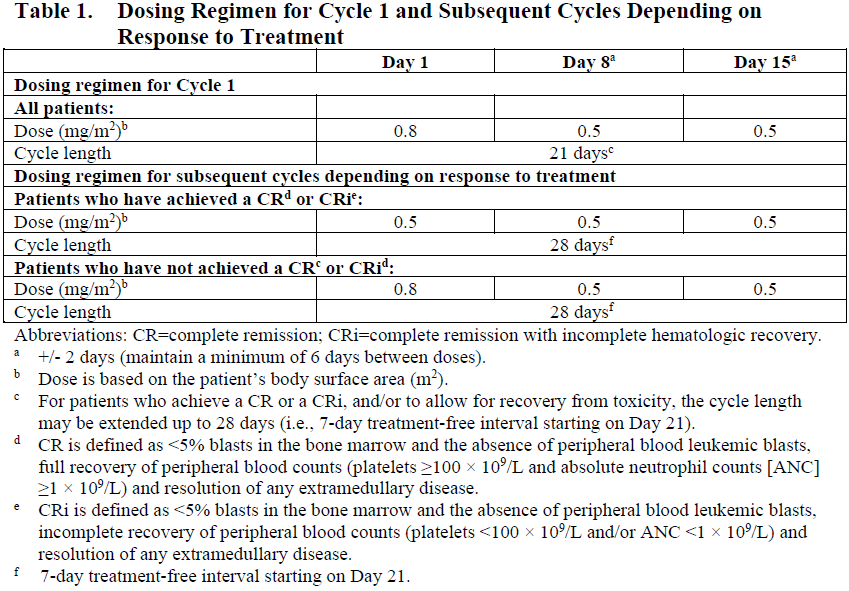 Dose modifications Dose modification of inotuzumab ozogamicin may be required based on individual safety and tolerability (see Section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Management of some adverse drug reactions may require dosing interruptions and/or dose reductions, or permanent discontinuation of inotuzumab ozogamicin (see Sections 4.4 and 4.8 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If the dose is reduced due to inotuzumab ozogamicin-related toxicity, the dose must not be re-escalated. Table 2 and Table 3 show the dose modification guidelines for hematologic and nonhematologic toxicities, respectively. Inotuzumab ozogamicin doses within a treatment cycle (i.e., Days 8 and/or 15) do not need to be interrupted due to neutropenia or thrombocytopenia, but dosing interruptions within a cycle are recommended for nonhematologic toxicities. 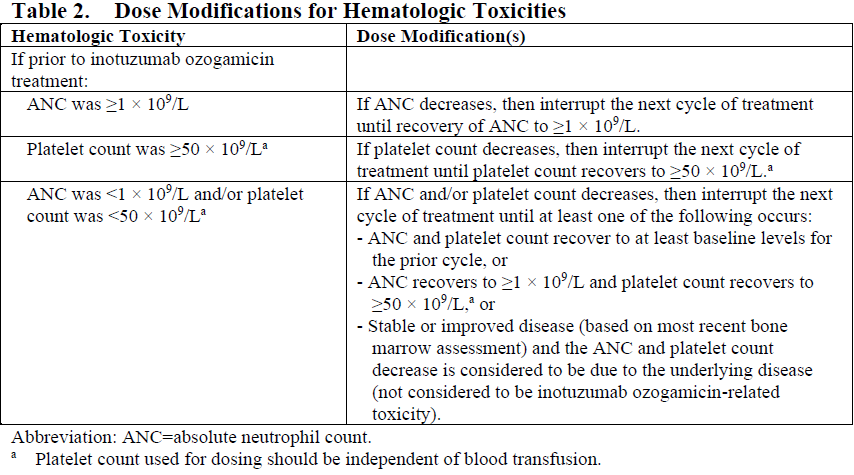 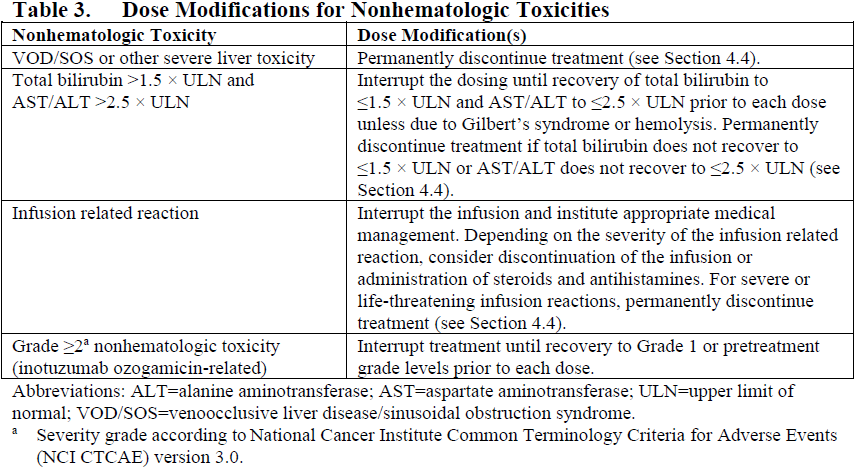 Table 4 shows the dose modification guidelines depending on the duration of dosing interruptions due to toxicity. 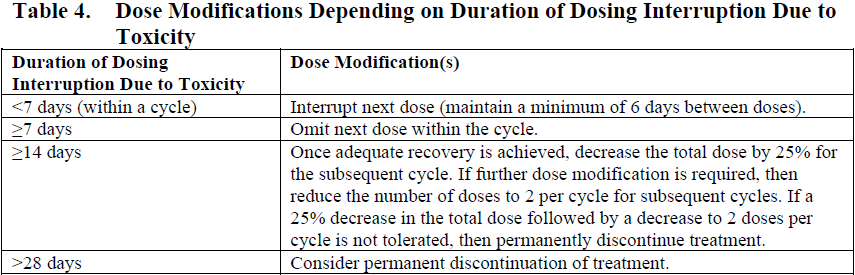 Special populations _Elderly patients_ No adjustment to the starting dose is required based on age (see Section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Increased age may be associated with an increased risk of venoocclusive liver disease/sinusoidal obstruction syndrome (VOD/SOS) after HSCT (see Section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Hepatic impairment_ No adjustment to the starting dose is required in patients with hepatic impairment defined by total bilirubin ≤1.5 × upper limit of normal (ULN) and aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ≤2.5 × ULN (see Section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). There is limited safety information available in patients with total bilirubin >1.5 × ULN and AST/ALT >2.5 × ULN prior to dosing. Interrupt dosing until recovery of total bilirubin to ≤1.5 × ULN and AST/ALT to ≤2.5 × ULN prior to each dose unless due to Gilbert’s syndrome or hemolysis. Permanently discontinue treatment if total bilirubin does not recover to ≤1.5 × ULN or AST/ALT does not recover to ≤2.5 × ULN (see Table 3 and Section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment_ No adjustment to the starting dose is required in patients with mild, moderate, or severe renal impairment (creatinine clearance \[CLcr\] 60–89 mL/min, 30–59 mL/min, or 15–29 mL/min, respectively) (see Section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The safety and efficacy of inotuzumab ozogamicin have not been studied in patients with end-stage renal disease. _Pediatric population_ The safety and efficacy of inotuzumab ozogamicin in the pediatric population (<18 years) have not been established. Currently available data are described in Sections 5.1 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ but no recommendation on a posology can be made. Method of administration Inotuzumab ozogamicin is for intravenous use. The infusion must be administered over 1 hour. Do not administer inotuzumab ozogamicin as an intravenous push or bolus. Inotuzumab ozogamicin must be reconstituted and diluted before administration. For instructions on reconstitution and dilution of inotuzumab ozogamicin before administration, see Section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAVENOUS
Medical Information
**4.1. Therapeutic indications** Inotuzumab ozogamicin is indicated for the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
**4.3. Contraindications** - Hypersensitivity to inotuzumab ozogamicin or to any of the excipients. - Patients who have ongoing VOD/SOS. - Patients with serious ongoing hepatic disease (e.g., cirrhosis, nodular regenerative hyperplasia, active hepatitis).
L01XC26
xl 01 xc 26
Manufacturer Information
PFIZER PRIVATE LIMITED
Wyeth Pharmaceutical Division of Wyeth Holding LLC
Active Ingredients
Documents
Package Inserts
Besponsa powder PI.pdf
Approved: July 2, 2020
