Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**4.2 Posology and method of administration** Posology 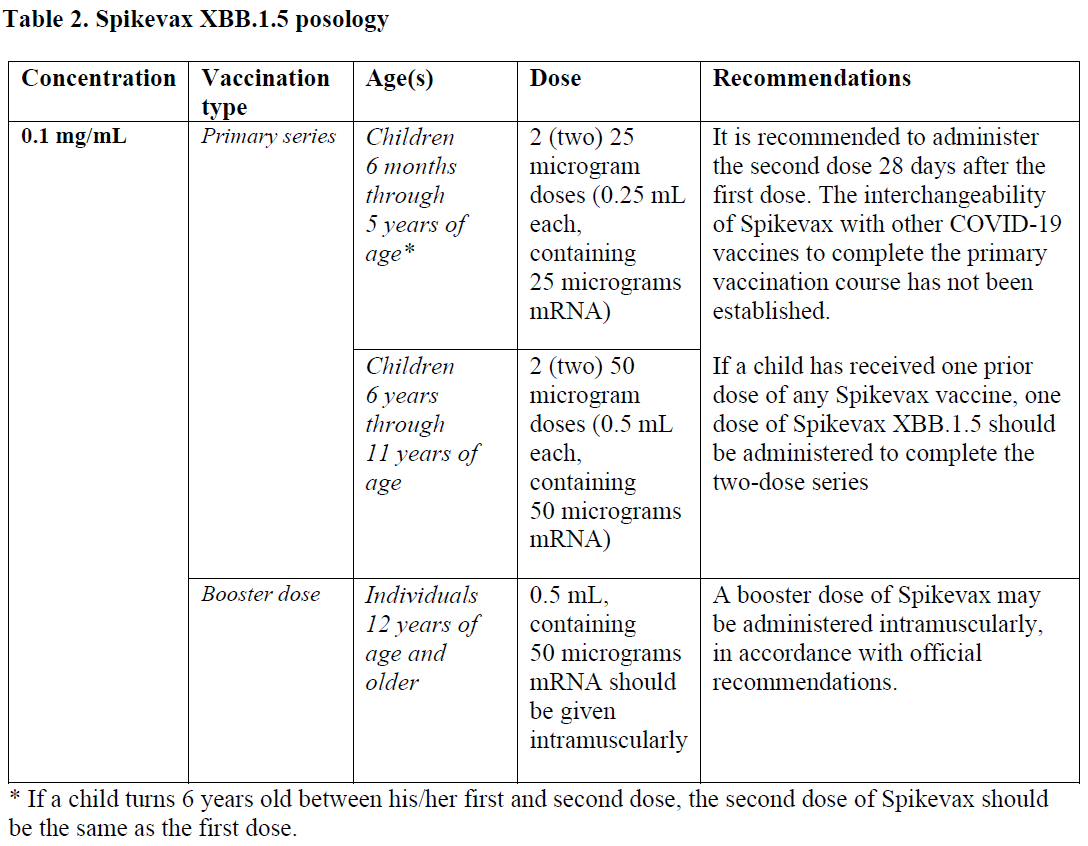 _Paediatric population_ The safety and efficacy of Spikevax XBB.1.5 in children less than 6 months of age have not yet been established. No data are available. _Elderly population_ No dosage adjustment is required in elderly individuals ≥65 years of age. Method of administration The vaccine should be administered intramuscularly. The preferred site is the deltoid muscle of the upper arm, or in infants and young children, the anterolateral aspect of the thigh. Do not administer this vaccine intravascularly, subcutaneously or intradermally. The vaccine should not be mixed in the same syringe with any other vaccines or medicinal products. For precautions to be taken before administering the vaccine, see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. For instructions regarding thawing, handling and disposal of the vaccine, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAMUSCULAR
Medical Information
**4.1 Therapeutic indications** Spikevax XBB.1.5 is indicated for active immunisation to prevent COVID-19 disease caused by SARS-CoV-2 in individuals 6 months of age and older (see sections 4.2 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The use of this vaccine should be in accordance with official recommendations.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
J07BN01
covid-19, RNA-based vaccine
Manufacturer Information
MODERNA BIOTECH SINGAPORE PTE. LTD.
Rovi Pharma Industrial Services, S.A.
Catalent Indiana, LLC
Lonza AG (manufacturer of SM-102 LNP, mRNA-1273 LNP-B)
ModernaTX, Inc. (manufacturer of SM-102 LNP, mRNA-1273 LNP-B)
