Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**Posology and Method of Administration** Treatment should be initiated and supervised by a physician experienced in the management of haemophilia. **Posology** The dosage and duration of the therapy depend on the severity of the disorder of haemostasis, on the location and the extent of bleeding and on the clinical condition of the patient. Dosage is independent of the patient‘s inhibitor titre. Since the response to treatment may differ from patient to patient the dosage recommendations are only guideline. Dosage and frequency of administration should always be guided by the clinical efficacy in the individual case. As a general guide a dose of 50 units to 100 units of FEIBA per kg body weight (bw.) is recommended. A single dose of 100 units/kg bw. and a daily dose of 200 units/kg bw. should not be exceeded. 01. **Spontaneous Haemorrhage** **Joint, Muscle and Soft Tissue Haemorrhage** For minor to moderate bleedings a dose of 50 – 75 units/kg bw. is recommended at 12-hour intervals. Treatment should be continued until clear signs of clinical improvement appear, such as relief of pain, reduction of swelling or mobilisation of the joint. For major muscle and soft tissue haemorrhage, such as retroperitoneal bleeding, a dose of 100 units/kg bw. at 12-hour intervals is recommended. **Mucous Membrane Haemorrhage** A dose of 50 units/kg bw. is recommended to be given every 6 hours with careful monitoring of the patient (visible bleeding site, repeated measurements of haematocrit). If the haemorrhage does not stop, the dose may be increased to 100 units/kg bw. (Do not exceed the maximum daily dose of 200 units/kg bw.) **Other Severe Haemorrhages** Severe haemorrhages, such as CNS bleedings have been effectively treated with doses of 100 units/kg bw. at 12-hour intervals. In individual cases FEIBA may be given at 6-hour intervals until clear clinical improvement is achieved. (Do not exceed the maximum daily dose of 200 units/kg bw.) 02. **Surgery** Taking care not to exceed the maximum daily dose, 50 – 100 units/kg bw. should be given at intervals of up to 6 hours. 03. **Prophylactic Treatment** For dosage recommendations for prophylactic treatment, see table 2. 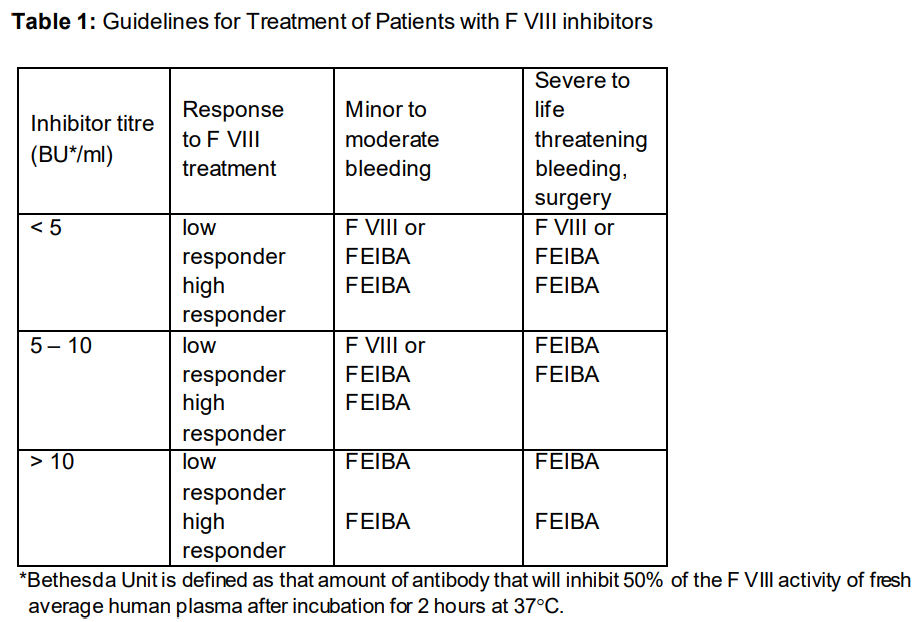 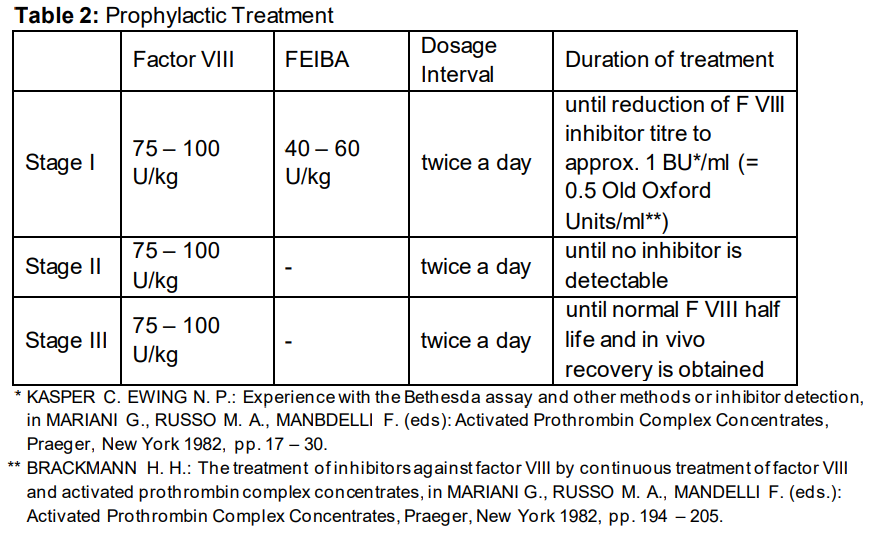 10. **Monitoring** In case of inadequate response to treatment with the product, it is recommended that a platelet count be performed because a sufficient number of functionality intact platelets are considered to be necessary for the efficacy of the product. Due to the complex mechanism of action, no direct monitoring of active ingredients is available. Coagulation tests such as whole blood clotting time (WBCT) and the aPTT may not correlate with clinical improvement. Global haemostatic tests such as thromboelastogram (TEG) or thrombin generation assay (TGA) may be useful tools to monitor and optimize the treatment. **Method of Administration** Reconstitute the product as described under “Instructions for Use and Handling, and Disposal” – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. FEIBA must be administered as an intravenous injection or infusion. The rate of administration should ensure the comfort of the patient and should not exceed a maximum of 2 units/kg bw. per minute.
INTRAVENOUS
Medical Information
**Therapeutic Indications** - Treatment and prophylaxis of bleeding in haemophilia A patients with F VIII inhibitor - Treatment and prophylaxis of bleeding in haemophilia B patients with F IX inhibitor - Treatment of bleeding in non-haemophiliacs with acquired inhibitors to factors VIII, XI, XII in cases of severe or life-threatening haemorrhages In one case, FEIBA was successfully used in a patient with an inhibitor, suffering from von Willebrand’s disease. FEIBA was also used in combination with Factor VIII concentrate for a long-term therapy to achieve a complete and permanent elimination of the F VIII inhibitor so as to allow for regular treatment with F VIII concentrate as in patients without inhibitor. For guidelines for treatment of patients with F VIII inhibitors see table 1 in section “Posology and Method of Administration, 3 Prophylactic Treatment”.
**Contraindications** FEIBA must not be used in the following situations if therapeutic alternatives to FEIBA are available. In the following situations FEIBA should only be used if– for example due to a very high inhibitor titre – no response to treatment with the appropriate coagulation factor concentrate can be expected. **Hypersensitivity to the product** **Disseminated Intravascular Coagulation (DIC):** - Laboratory and/or clinical symptoms, which are clearly indicative of DIC. - Laboratory, histological and/or clinical signs of liver damage; due to the delayed clearance of activated coagulation factors such patients are at an increased risk of developing DIC. **Myocardial Infarction, Acute Thrombosis and/or Embolism:** In patients with a tentative or definite diagnosis of coronary heart disease as well as in patients with acute thrombosis and/or embolism the use of FEIBA is only indicated in life-threatening bleeding episodes.
B02BD01
coagulation factor IX, II, VII and X in combination
Manufacturer Information
TAKEDA PHARMACEUTICALS (ASIA PACIFIC) PTE. LTD.
Takeda Manufacturing Austria AG
Siegfried Hameln GmbH (Solvent)
Active Ingredients
Documents
Package Inserts
FEIBA PI_Approved.pdf
Approved: April 11, 2022
