Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**Posology and Method of Administration** ZALTRAP should be administered under the supervision of a physician experienced in the use of antineoplastic medicinal products. Posology The recommended dose of ZALTRAP, administered as an intravenous infusion over 1 hour, is 4 mg/kg of body weight, followed by the FOLFIRI regimen. This is considered as one treatment cycle. The FOLFIRI regimen to be used is irinotecan 180 mg/m2 intravenous infusion over 90 minutes and folinic acid (dl racemic) 400 mg/m2 intravenous infusion over 2 hours at the same time on day 1 using a Y line, followed by 5-fluorouracil (5-FU) 400 mg/m2 intravenous bolus, followed by 5-FU 2400 mg/m2 continuous intravenous infusion over 46 hours. The treatment cycle is repeated every 2 weeks. ZALTRAP treatment should be continued until disease progression or unacceptable toxicity occurs. _Dose Modification_ ZALTRAP should be discontinued for (see section Special Warnings and Precautions for Use – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_): - Severe haemorrhage - Gastrointestinal (GI) perforation - Fistula formation - Hypertension that is not adequately controlled with anti-hypertensive therapy or occurrence of hypertensive crisis or hypertensive encephalopathy - Cardiac failure and ejection fraction decreased - Arterial thromboembolic events (ATE) - Grade 4 venous thromboembolic events (including pulmonary embolism) - Nephrotic syndrome or thrombotic microangiopathy (TMA) - Severe hypersensitivity reactions (including bronchospasm, dyspnoea, angioedema, and anaphylaxis) (see sections Contraindications and Special Warnings and Precautions for Use – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) - Compromised wound healing requiring medical intervention - Posterior reversible encephalopathy syndrome (PRES) (also known as reversible posterior leukoencephalopathy syndrome (RPLS)) ZALTRAP should be temporarily suspended for at least 4 weeks prior to elective surgery (see section Special Warnings and Precautions for Use – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 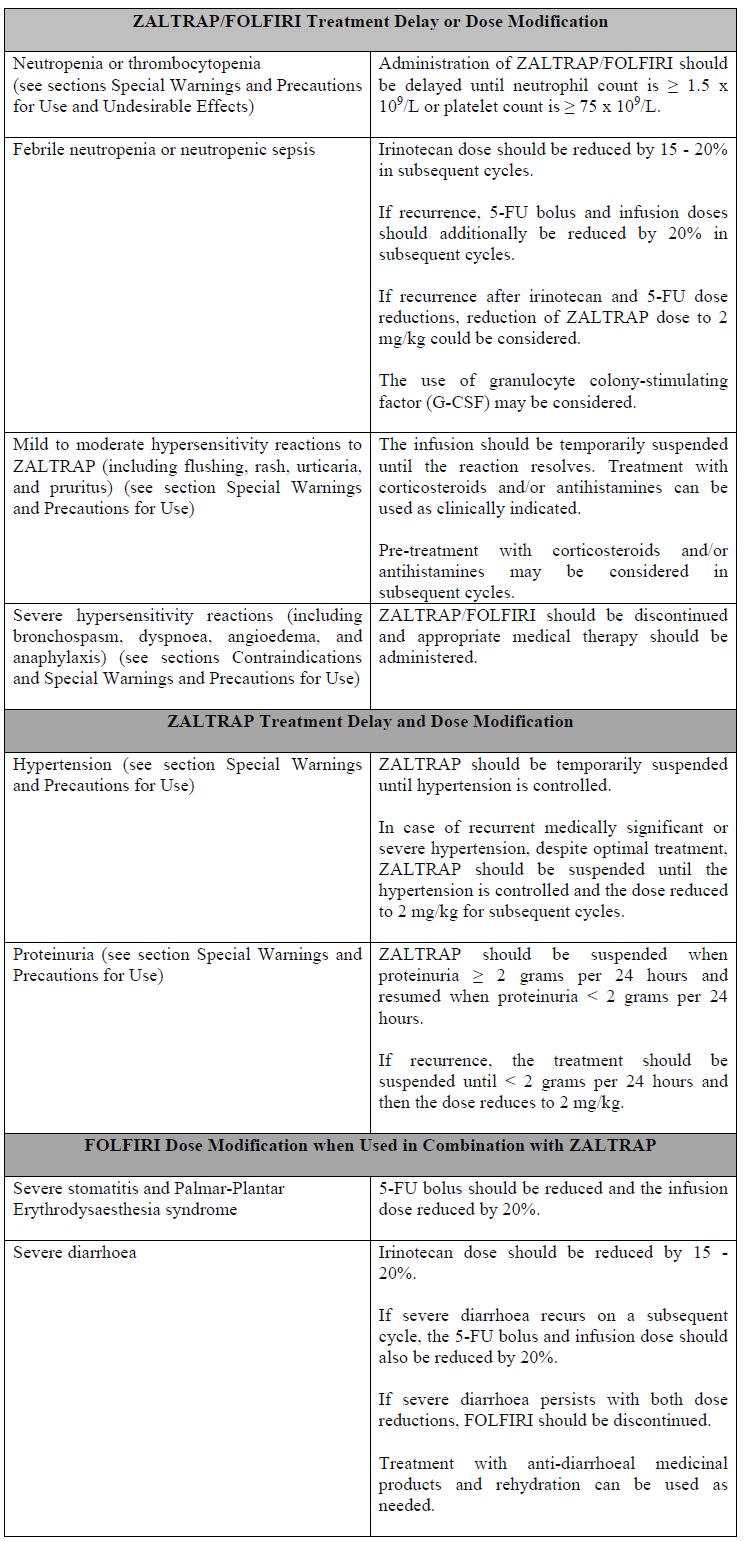 For additional toxicities related to irinotecan, 5-FU, or folinic acid, refer to the current respective summary of product characteristics. Special Populations _Older People_ In the pivotal MCRC study, 28.2% of patients were aged ≥ 65 and < 75 and 5.4% of patients were aged ≥ 75. No dose adjustments of ZALTRAP is required in the older people. _Hepatic Impairment_ There have been no formal studies with ZALTRAP in patients with hepatic impairment (see section Pharmacokinetic Properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Clinical data suggest that no change in aflibercept dose is required in patients with mild to moderate hepatic impairment. There are no data regarding the administration of aflibercept in patients with severe hepatic impairment. _Renal Impairment_ There have been no formal studies with ZALTRAP in patients with renal impairment (see section Pharmacokinetic Properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Clinical data suggest that no change in starting dose is required in patients with mild to moderate renal impairment. There are very limited data in patients with severe renal impairment; therefore, these patients should be treated with caution. _Paediatric Population_ There is no relevant use of ZALTRAP in the paediatric population for the indication of metastatic colorectal cancer. Method of Administration ZALTRAP is to be administered only as an intravenous infusion over 1 hour. Due to hyperosmolality (1000 mOsmol/kg) of the ZALTRAP concentrate, undiluted ZALTRAP concentrate must not be administered as an intravenous push or bolus. ZALTRAP must not be administered as an intravitreal injection (see sections Contraindications and Special Warnings and Precautions for Use – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Each vial of concentrate for solution for infusion is for single use (single-dose) only. Diluted solutions of ZALTRAP should be administered using infusion sets containing a 0.2 micron polyethersulfone filter. The infusion sets should be made of one of the following materials: - polyvinyl chloride (PVC) containing bis(2-ethylhexyl) phthalate (DEHP) - DEHP free PVC containing trioctyl-trimellitate (TOTM) - polypropylene - polyethylene lined PVC - polyurethane Filters made of polyvinylidene fluoride (PVDF) or nylon must not be used. _Precautions to be taken before handling or administering the medicinal product_ For instructions on dilution of the medicinal product before administration, see section Special Precautions for Disposal and Other Handling – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAVENOUS
Medical Information
**Therapeutic Indications** ZALTRAP in combination with irinotecan/5-fluorouracil/folinic acid (FOLFIRI) chemotherapy is indicated in adults with metastatic colorectal cancer (MCRC) that is resistant to or has progressed after an oxaliplatin-containing regimen.
**Contraindications** Hypersensitivity to aflibercept or to any of the excipients listed in section List of Excipients – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Ophthalmic / intravitreal use due to hyperosmotic properties of ZALTRAP (see section Special Warnings and Precautions for Use – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For contraindications related to FOLFIRI components (irinotecan, 5-FU, and folinic acid), refer to the current respective summary of product characteristics.
Pending
xpending
Manufacturer Information
SANOFI-AVENTIS SINGAPORE PTE. LTD.
Sanofi-Aventis Deutschland GmbH
Active Ingredients
Documents
Package Inserts
Zaltrap Concentrate for Solution for Infusion PI.pdf
Approved: February 25, 2020
