Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**Dosage and Administration** RYBREVANT® should be administered by a healthcare professional with appropriate medical support to manage infusion-related reactions (IRRs) if they occur (see _Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Administer pre-infusion medications (see _Dosage and Administration – Pre-infusion medications_). When considering the use of RYBREVANT®, EGFR Exon 20 insertion mutation presence should be established using a validated test (see _Pharmacodynamic Effects – Clinical studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Dosage – adults (≥18 years)** The recommended dose of RYBREVANT® is provided in Table 1, and the dosing schedule is provided in Table 2 (see _Infusion Rates – Table 4_). 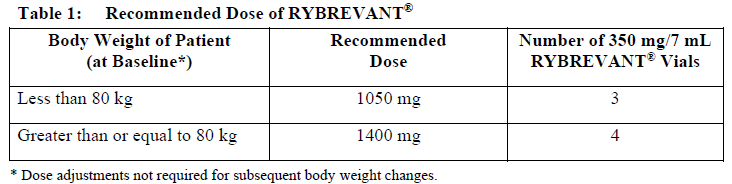  It is recommended that patients are treated with RYBREVANT® until unacceptable toxicity or disease progression. _**Pre-infusion medications**_ Prior to initial infusion of RYBREVANT® (Week 1, Days 1 and 2), administer antihistamines, antipyretics, and glucocorticoids to reduce the risk of IRRs. For subsequent doses, administer antihistamines and antipyretics. Administer antiemetics as needed. 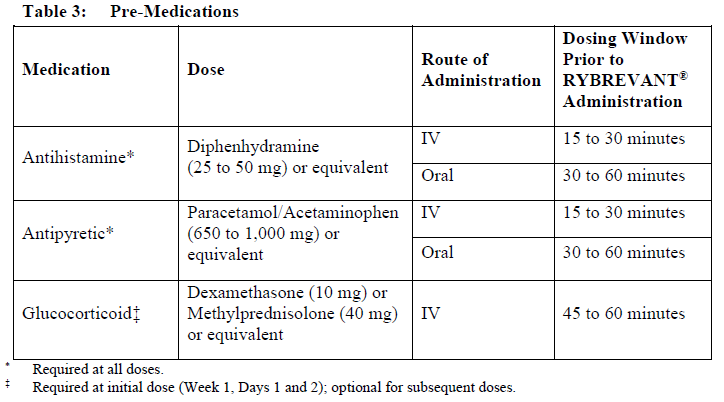 _**Infusion Rates**_ Administer RYBREVANT® infusion intravenously according to the infusion rates in Table 4. Due to the frequency of IRRs at the first dose, infusion via a peripheral vein at Week 1 and Week 2 should be considered to minimize drug exposure in the event of an IRR; infusion via central line may be administered for subsequent weeks. It is recommended for the first dose to be diluted as close to administration as possible to allow for maximal flexibility in IRR management. 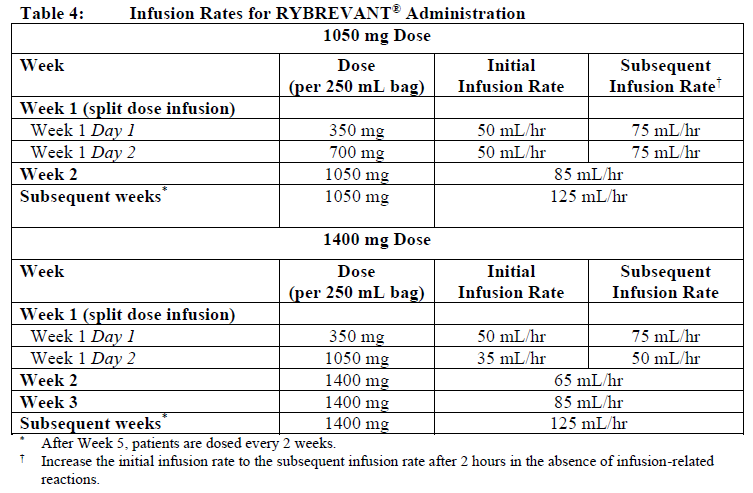 **Missed dose(s)** If a planned dose of RYBREVANT® is missed, the dose should be administered as soon as possible and the dosing schedule should be adjusted accordingly, maintaining the treatment interval. **Dose modifications** The recommended dose reductions for adverse reactions (see Table 6) are listed in Table 5. 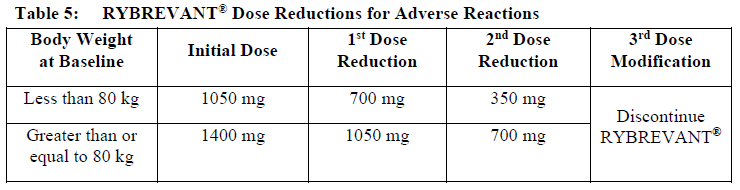 The recommended dosage modifications for adverse reactions are provided in Table 6. 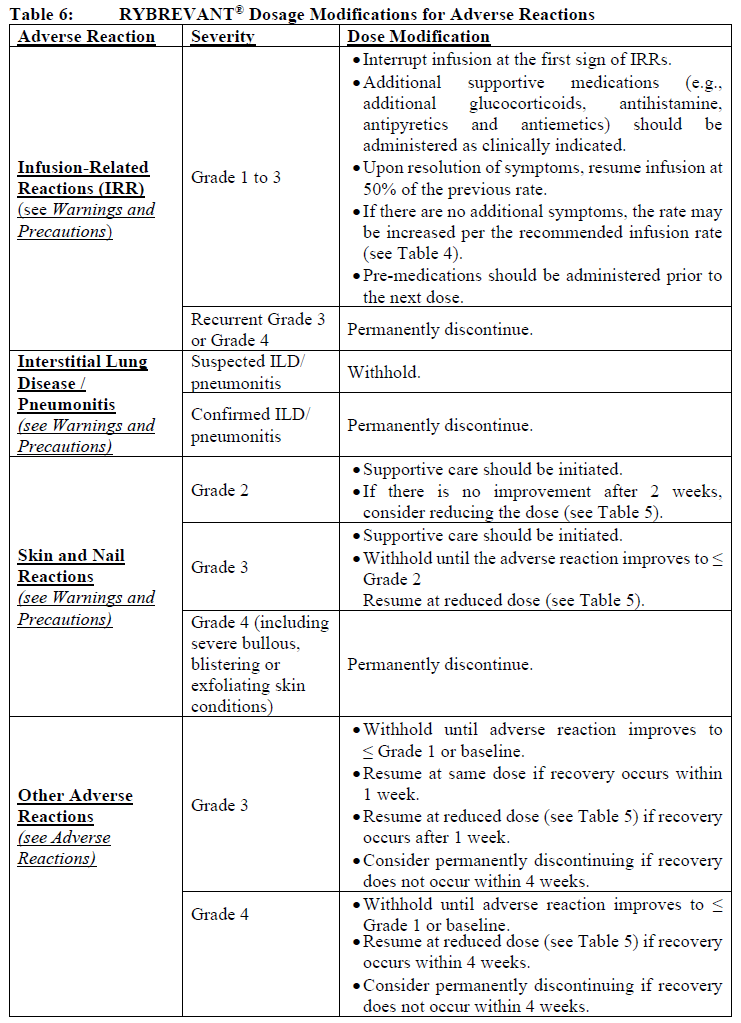 **Special populations** _**Pediatrics (17 years of age and younger)**_ The safety and efficacy of RYBREVANT® have not been established in pediatric patients. _**Elderly (65 years of age and older)**_ Of the 380 patients treated with RYBREVANT® in EDI1001, 41% were 65 years of age or older, and 11% were 75 years of age or older. No overall differences in safety or effectiveness were observed between patients ≥ 65 years of age and patients < 65 years of age. No dosage adjustment is necessary (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Renal impairment**_ No formal studies of amivantamab in patients with renal impairment have been conducted. Based on population pharmacokinetic (PK) analyses, no dosage adjustment is necessary for patients with mild or moderate renal impairment. No data are available in patients with severe renal impairment (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Hepatic impairment**_ No formal studies of amivantamab in patients with hepatic impairment have been conducted. Based on population PK analyses, no dosage adjustment is necessary for patients with mild hepatic impairment. No data are available in patients with moderate or severe hepatic impairment (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Administration** _**Preparation for administration**_ RYBREVANT® solution must be diluted and prepared for intravenous infusion by a healthcare professional using aseptic technique. 1. Determine the dose required (either 1050 mg or 1400 mg) and number of RYBREVANT® vials needed based on patient’s baseline weight (see _Dosage_). Each vial of RYBREVANT® contains 350 mg of amivantamab. 2. Check that the RYBREVANT® solution is colorless to pale yellow. Do not use if discoloration or visible particles are present. 3. Withdraw and then discard a volume of either 5% dextrose \[glucose\] solution or 0.9% sodium chloride solution from the 250 mL infusion bag equal to the volume of RYBREVANT® to be added (i.e., discard 7 mL diluent from the infusion bag for each RYBREVANT® vial). Infusion bags must be made of polyvinylchloride (PVC), polypropylene (PP), polyethylene (PE), or polyolefin blend (PP+PE). 4. Withdraw 7 mL of RYBREVANT® from each vial and add it to the infusion bag. The final volume in the infusion bag should be 250 mL. Each vial contains a 0.5 mL overfill to ensure sufficient extractable volume. Discard any unused portion left in the vial. 5. Gently invert the bag to mix the solution. Do not shake. 6. Visually inspect the diluted solution before administration. Do not use if discoloration or visible particles are observed. 7. Diluted solutions should be administered within 10 hours (including infusion time) at room temperature (15°C to 25°C) and in room light. _**Administration**_ 1. Administer the diluted solution by intravenous infusion using an infusion set fitted with a flow regulator and with an in-line, sterile, non-pyrogenic, low protein-binding polyethersulfone (PES) filter (pore size 0.2 micrometer). Administration sets must be made of either polyurethane (PU), polybutadiene (PBD), PVC, PP, or PE. 2. Do not infuse RYBREVANT® concomitantly in the same intravenous line with other agents. 3. This medicinal product is for single use only. Any unused medicinal product should be disposed of in accordance with local requirements.
INTRAVENOUS
Medical Information
**Indications** RYBREVANT® is indicated for the treatment of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with activating epidermal-growth factor receptor (EGFR) Exon 20 insertion mutations whose disease has progressed on or after platinum-based chemotherapy.
**Contraindications** Hypersensitivity to the active substance(s) or to any of the excipients listed in _List of Excipients_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
L01FX18
amivantamab
Manufacturer Information
JOHNSON & JOHNSON INTERNATIONAL (SINGAPORE) PTE. LTD.
Cilag AG
Active Ingredients
Documents
Package Inserts
Rybrevant Concentrate For Solution For Infusion PI.pdf
Approved: April 28, 2023
