Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
**4.2 Posology and method of administration** The immunisation schedules for Rabipur should be based on official recommendations. _Posology_ The recommended single intramuscular (IM) dose is 1.0 ml in individuals of all ages. _PRE-EXPOSURE PROPHYLAXIS (PrEP)_ Pre-exposure prophylaxis is recommended for anyone who is at continual, frequent or increased risk for exposure to the rabies virus, as a result of their residence or occupation, such as laboratory workers dealing with rabies virus and other lyssaviruses, veterinarians and animal handlers. Travellers in high-risk areas should be vaccinated after a risk assessment. Children living in or visiting rabies-affected areas are at particular risk and should be given pre-exposure prophylaxis on an individual basis or in mass campaigns when there are no economic, programmatic or logistical obstacles. _Primary immunisation_ In previously unvaccinated individuals, an initial course of PrEP consists of three doses (each of l.0 ml) administered IM, according to the conventional or rapid regimen, as follows: 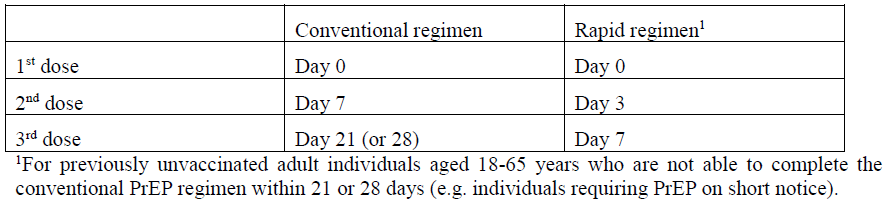 _Booster_ Rabipur may be used for booster vaccination after prior immunisation with human diploid cell rabies vaccine (HDCV). The need of intermittent serological testing for the presence of antibody ≥ 0.5 international units/ml and the administration of booster doses should be assessed in accordance with official recommendations. The following provides general guidance: - Testing for neutralising antibodies at 6 month intervals is usually recommended if the risk of exposure is high (e.g. laboratory staff working with rabies vaccine) - In persons who are considered to be at continuing risk of exposure to rabies (e.g. veterinarians and their assistants, wildlife workers, hunters), a serological test should usually be performed at least every 2 years, with shorter intervals if appropriate to the perceived degree of risk. A booster would be recommended only if Rabies Virus Neutralising Antibody (RVNA) concentration falls to less than 0.5 international units/ml (assessed by rapid fluorescent focus inhibition test \[RFFIT\]). Alternatively, booster doses may be given at official recommended intervals without prior serological testing according to the perceived risk. Experience shows that booster doses are generally required every 2–5 years. The individual IM booster dose is 1.0 ml. _POST-EXPOSURE PROPHYLAXIS PEP)_ Post-exposure prophylaxis according to the WHO recommendations consists of - local treatment of the wound as soon as possible after exposure, - a course of rabies vaccine that meets WHO recommendations - administration of rabies immunoglobulin, if indicated. The indication for post-exposure prophylaxis (PEP) depends on the type of contact with the suspected rabid animal, as provided in the Table below. Post-exposure immunisation should begin as soon as possible after exposure and should be accompanied by local measures to the site of inoculation so as to reduce the risk of infection. Official guidance according to WHO PEP prophylaxis guideline should be sought regarding the appropriate concomitant measures that should be taken to prevent establishment of infection (see also section 4.4 Special warnings and special precautions for use – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 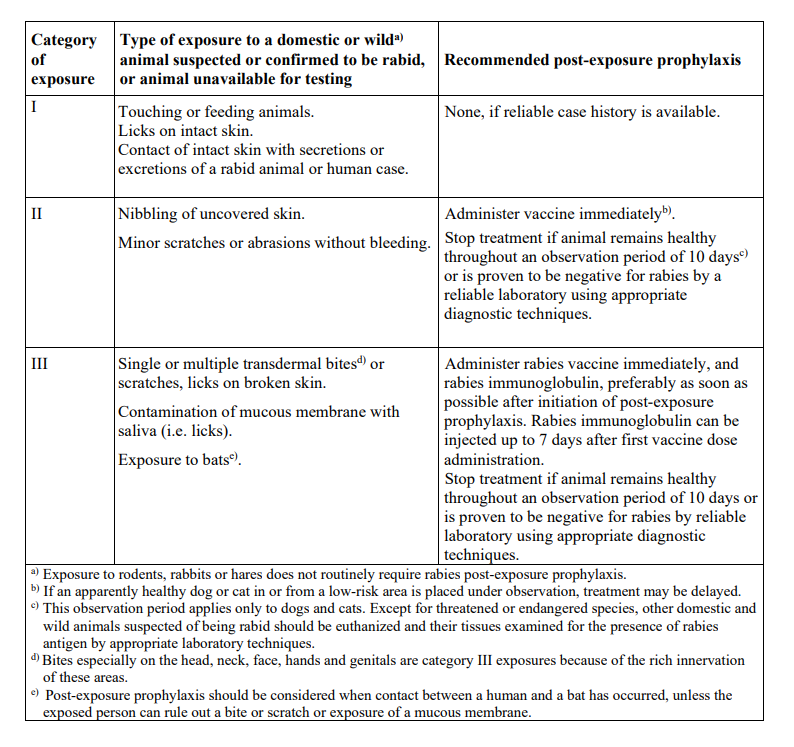 _Previously unvaccinated individuals_ The dose for intramuscular injection is 1.0 ml, according to the following regimens: 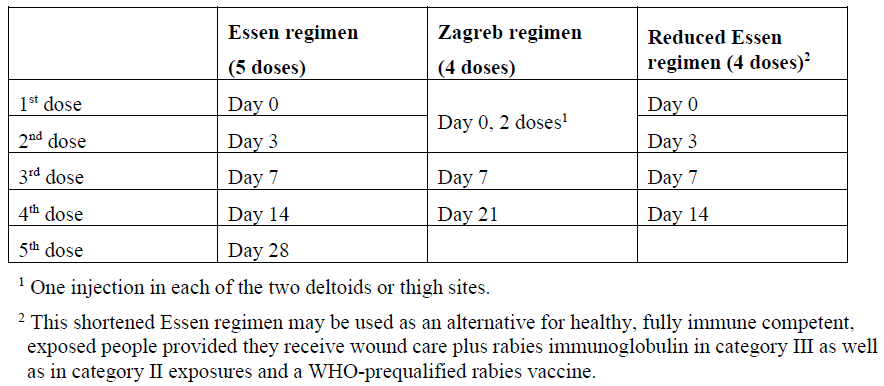 _Previously vaccinated individuals_ In previously vaccinated individuals, post-exposure prophylaxis consists of two doses administered as follows: 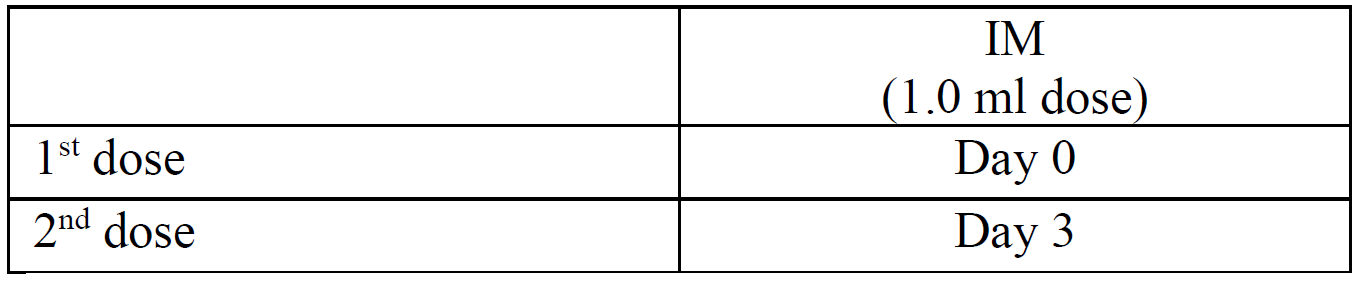 Rabies immunoglobulin is not indicated in such cases. _Dosing in different populations:_ _Post-exposure prophylaxis (PEP) for immunocompromised individuals_ For category II and III exposures, a complete series of 5 doses is required in combination with comprehensive wound management and local infiltration of rabies immunoglobulin, according to the following regimens: 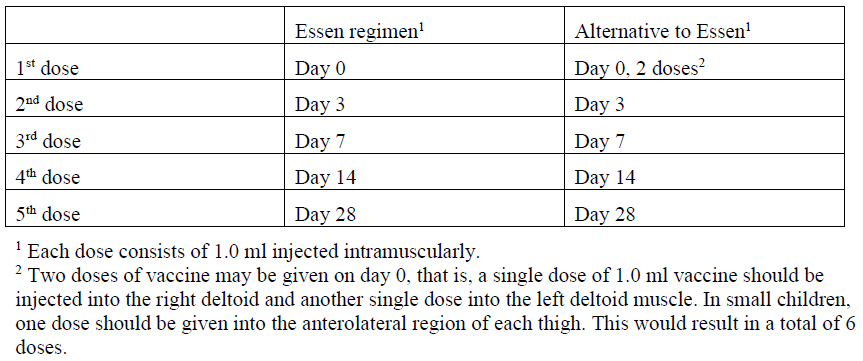 Severely immunosuppressed patients may not develop an immunologic response after rabies vaccination. In immunocompromised patients, the neutralising antibody titre should be measured 14 days after the first injection. Patients with a titre that is less than 0.5 international units/ml should be given another two doses of vaccine simultaneously and as soon as possible. Further checks on the antibody titre should be made and further doses of vaccine should be administered as necessary. Immunosuppressive agents should not be administered during post-exposure therapy unless essential for the treatment of other conditions. In all cases, the immunisation schedule must be followed exactly as recommended, even if the patient does not present for treatment until a considerable time has elapsed since exposure. _Method of Administration_ For adults and children ≥ 2 years of age, the vaccine should be given by intramuscular injection into the deltoid muscle; For children < 2 years, the anterolateral region of the thigh is recommended. For instructions on reconstitution of the medicinal product before administration, see Section 6.5 Instructions for use and handling – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAMUSCULAR
Medical Information
**4.1 Therapeutic indications** Rabipur is indicated for active immunization against rabies in individuals of all ages. This includes pre-exposure prophylaxis (i.e. before possible risk of exposure to rabies), in both primary series and booster dose, and post-exposure prophylaxis (i.e. after suspected or proven exposure to rabies).
**4.3 Contraindications** Pre-exposure prophylaxis Rabipur should not be administered to subjects with a known hypersensitivity to any of the components of the vaccine (see sections 2. Qualitative and quantitative composition and 6.1 List of excipients – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Individuals with acute diseases requiring treatment should not be vaccinated until at least 2 weeks after recovery. The presence of a minor infection, such as a cold, should not result in the deferral of vaccination. Post-exposure prophylaxis (PEP) In view of the almost invariably fatal outcome of rabies, no contraindication to post-exposure prophylaxis is indicated, including pregnancy.
J07BG01
rabies, inactivated, whole virus
Manufacturer Information
AENON PHARMACEUTICALS SEA PTE. LTD.
GSK Vaccines GmbH (bulk product, filling, lyophilisation, QC testing)
GSK Vaccines S.r.l. (water for injection)
Vetter Pharma-Fertigung GmbH & Co. KG (water for injection in PFS)
Active Ingredients
Documents
Package Inserts
1.4.3 Proposed clean_PPC-0026188_Market Produced Artwork_CO-0039076_V01[3].pdf
Approved: June 23, 2021
