Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Posology and method of administration** Treatment with TAGRISSO should be initiated by a physician experienced in the use of anticancer therapies. When considering the use of TAGRISSO, EGFR mutation status should be determined using a validated test method (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) for: - exon 19 deletions or exon 21 (L858R) substitution mutations (in tumour specimens for adjuvant treatment and tumour or plasma specimens for first-line treatment). - T790M mutations (in tumour or plasma specimens following progression on or after EGFR TKI therapy). **Posology** The recommended dose of TAGRISSO is 80 mg osimertinib once a day. **Duration of treatment** Patients in the adjuvant setting should receive treatment until disease recurrence or unacceptable toxicity or up to a maximum of 3 years. Treatment duration for more than 3 years was not studied. Patients with locally advanced or metastatic lung cancer should receive treatment until disease progression or unacceptable toxicity. **Missed dose** If a dose of TAGRISSO is missed, make up the dose unless the next dose is due within 12 hours. TAGRISSO can be taken with or without food at the same time each day. **Dose adjustments** Dosing interruption and/or dose reduction may be required based on individual safety and tolerability. If dose reduction is necessary, then the dose of TAGRISSO should be reduced to 40 mg taken once daily. Dose reduction guidelines for adverse reactions toxicities are provided in Table 1. 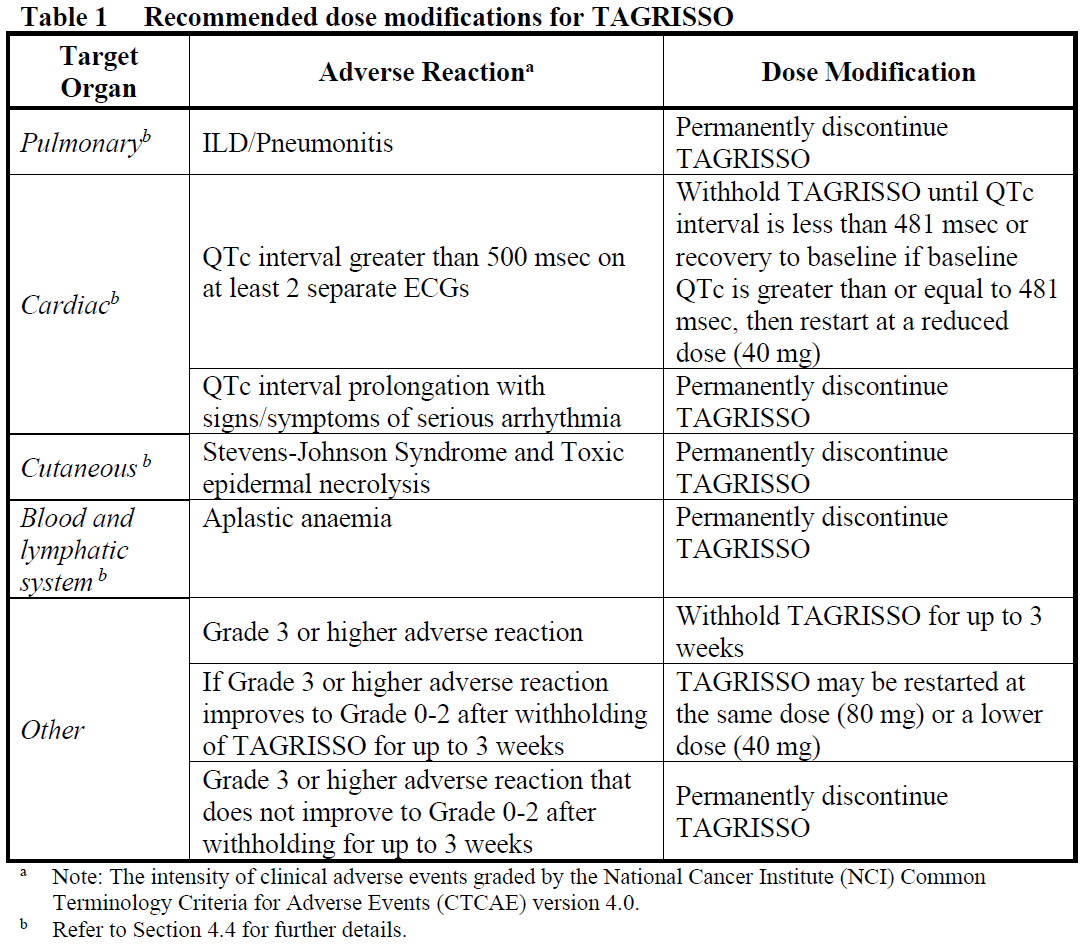 **Special patient populations** No dosage adjustment is required due to patient age, body weight, gender, ethnicity and smoking status (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Paediatric and adolescents** The safety and efficacy of TAGRISSO in children or adolescents aged less than 18 years have not been established. No data are available. **Method of administration** This medicinal product is for oral use. The tablet should be swallowed whole with water. The tablet should not be crushed, split or chewed. If the patient is unable to swallow the tablet, it may first be dispersed in 50 mL of non-carbonated water. The tablet should be dropped in the water, without crushing, stirred until dispersed and immediately swallowed. An additional half a glass of water should be added to ensure that no residue remains and then immediately swallowed. No other liquids should be added. If administration via nasogastric tube is required, the same process as above should be followed but using volumes of 15 mL for the initial dispersion and 15 mL for the residue rinses. The resulting 30 mL of liquid should be administered as per the naso-gastric tube manufacturer’s instructions with appropriate water flushes. The dispersion and residues should be administered within 30 minutes of the addition of the tablets to water. **Elderly (>65 years)** Population pharmacokinetic (PK) analysis indicated that age did not have an impact on the exposure of osimertinib and hence, osimertinib can be used in adults without regard to age. **Hepatic impairment** Based on clinical studies, no dose adjustments are necessary in patients with mild hepatic impairment (Child Pugh A) or moderate hepatic impairment (Child Pugh B). Similarly based on population PK analysis, no dose adjustment is recommended in patients with mild hepatic impairment (total bilirubin ≤ULN and AST >ULN or total bilirubin between 1.0 to 1.5x ULN and any AST) or moderate hepatic impairment (total bilirubin between 1.5 to 3 times ULN and any AST). The appropriate dose of TAGRISSO has not been established in patients with severe hepatic impairment (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Renal impairment** Based on clinical studies and population PK analysis, no dose adjustments are necessary in patients with mild, moderate, or severe renal impairment. The safety and efficacy of TAGRISSO has not been established in patients with end-stage renal disease \[Creatinine clearance (CLcr) less than 15 mL/min, calculated by the Cockcroft and Gault equation\], or on dialysis. Caution should be exercised when treating patients with severe and end-stage renal impairment (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
ORAL
Medical Information
**4.1 Therapeutic indications** TAGRISSO (osimertinib) is indicated for: - the adjuvant treatment after tumour resection in patients with non-small cell lung cancer (NSCLC) whose tumours have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations. - the first-line treatment of patients with locally advanced or metastatic NSCLC whose tumours have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations. - the treatment of patients with locally advanced or metastatic EGFR T790M mutation-positive NSCLC whose disease has progressed on or after EGFR TKI therapy. (See Clinical efficacy and safety section – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_)
**4.3 Contraindications** None.
L01XE35
xl 01 xe 35
Manufacturer Information
ASTRAZENECA SINGAPORE PTE LTD
AstraZeneca AB
Active Ingredients
Documents
Package Inserts
1.4.3c Tagrisso PI V18.0_Proposed clean.pdf
Approved: January 26, 2023
