Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**4.2 Posology and method of administration** **Posology** Adults A low initial dose is recommended, and this must be adjusted according to the patient’s response in order to determine the minimal effective dose (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The dose recommendations for HALOPERIDOL INJECTION BP are presented in Table 1. 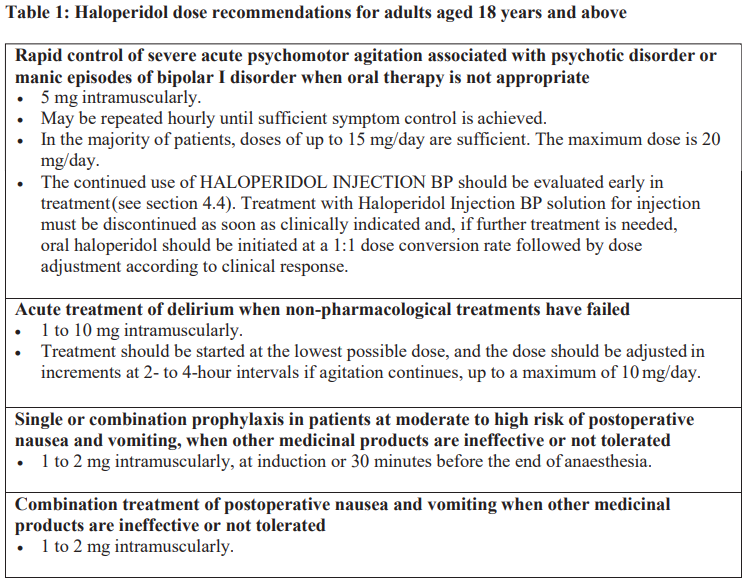 Treatment withdrawal Gradual withdrawal of haloperidol is advisable (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Special populations _Elderly_ The recommended initial haloperidol dose in elderly patients is half the lowest adult dose. Further doses may be administered and adjusted according to the patient’s response. Careful and gradual dose up-titration in elderly patients is recommended. The maximum dose is 5 mg/day. Doses above 5 mg/day should only be considered in patients who have tolerated higher doses and after reassessment of the patient’s individual benefit-risk profile. _Renal impairment_ The influence of renal impairment on the pharmacokinetics of haloperidol has not been evaluated. No dose adjustment is recommended, but caution is advised when treating patients with renal impairment. However, patients with severe renal impairment may require a lower initial dose, with further doses administered and adjusted according to the patient’s response (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Hepatic impairment_ The influence of hepatic impairment on the pharmacokinetics of haloperidol has not been evaluated. Since haloperidol is extensively metabolised in the liver, it is recommended to halve the initial dose. Further doses may be administered and adjusted according to the patient’s response (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Paedriatic population_ The safety and efficacy of HALOPERIDOL INJECTION BP in children and adolescents below 18 years of age have not been established. No data are available. **Method of administration** HALOPERIDOL INJECTION BP is recommended for intramuscular use only (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
INTRAMUSCULAR
Medical Information
**4.1 Therapeutic indications** HALOPERIDOL INJECTION BP is indicated in adult patients for: - Rapid control of severe acute psychomotor agitation associated with psychotic disorder or manic episodes of bipolar I disorder when oral therapy is not appropriate. - Acute treatment of delirium when non-pharmacological treatments have failed. - Single or combination prophylaxis in patients at moderate to high risk of postoperative nausea and vomiting, when other medicinal products are ineffective or not tolerated. - Combination treatment of postoperative nausea and vomiting when other medicinal products are ineffective or not tolerated.
**4.3 Contraindications** - Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. - Comatose state. - Central nervous system (CNS) depression. - Parkinson’s disease. - Dementia with Lewy bodies. - Progressive supranuclear palsy. - Known QTc interval prolongation or congenital long QT syndrome. - Recent acute myocardial infarction. - Uncompensated heart failure. - History of ventricular arrhythmia or torsades de pointes. - Uncorrected hypokalaemia. - Concomitant treatment with medicinal products that prolong the QT interval (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
N05AD01
haloperidol
Manufacturer Information
DUOPHARMA (SINGAPORE) PTE. LTD.
PANPHARMA GMBH
Active Ingredients
Documents
Package Inserts
Haloperidol Injection PI.pdf
Approved: January 10, 2022
