Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**Dosage regimen and administration** **Dosage regimen and method of administration** Tykerb should only be initiated by a physician experienced in the administration of anti-cancer agents. Prior to the initiation of treatment, left ventricular ejection fraction (LVEF) must be evaluated to ensure that baseline LVEF is within the institutional limits of normal (see Warnings and precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). LVEF must continue to be monitored during treatment with Tykerb to ensure that it does not fall below the institutional lower limit of normal (see Dosage regimen and administration – Dose delay and dose reduction – Cardiac events). Tykerb should be taken at least 1 hour before, or at least 1 hour after food (see Interactions – Drug food interaction and Clinical pharmacology – Pharmacokinetics – Absorption – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The recommended daily Tykerb dose should not be divided. Missed doses should not be replaced and the dosing should resume with the next scheduled daily dose (see Overdosage – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The full prescribing information of the co-administered medicinal product should be consulted for details of its posology, and safety information. **General target population** **Tykerb in combination with capecitabine** The recommended dose of Tykerb is 1250 mg (i.e. 5 tablets) once daily continuously when taken in combination with capecitabine. The recommended dose of capecitabine is 2000 mg/m2/day taken in 2 doses 12 hours apart on days 1–14 in a 21 day cycle (see Clinical studies – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Capecitabine should be taken with food or within 30 minutes after food. HER2 protein overexpression or gene amplification is necessary for the selection of patients for whom Tykerb therapy is appropriate. Evidence of a previous positive result for HER2 overexpression or gene amplification should be confirmed before initiating therapy with Tykerb. If are not available, repeat HER2 testing should be considered. Assessment of HER2 overexpression and/or of HER2 gene amplification should be performed by laboratories with accreditation or demonstrated proficiency. HER2 overexpressing tumours are defined by a score of 3+ using an immunohistochemistry (IHC)-based assessment, or IHC2+ and gene amplification or gene amplification alone. Treatment with Tykerb should be continued until disease progression or unacceptable toxicity occurs. **Tykerb in combination with an aromatase inhibitor** The recommended dose of Tykerb is 1500 mg (i.e. 6 tablets) once daily continuously when taken in combination with an aromatase inhibitor. When Tykerb is co-administered with the aromatase inhibitor letrozole, the recommended dose of letrozole is 2.5 mg once daily. If Tykerb is co-administered with an alternative aromatase inhibitor, please refer to the full prescribing information of the medicinal product for dosing details. **Dose delay and dose reduction (all indications)** **Cardiac events** (see Warnings and precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) Tykerb should be interrupted in patients with symptoms associated with decreased LVEF that are National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) grade 3 or greater or if their LVEF drops below the institutionals LLN. Tykerb may be restarted at a lower dose (reduced from 1250 mg/day to 1000 mg/day or from 1500 mg/day to 1250 mg/day) after a minimum of 2 weeks and if LVEF recovers to normal and the patient is asymptomatic. Based on current data, the majority of LVEF decreases occur within the first 9 weeks of treatment, however, there is limited data on long term exposure. **Interstitial lung disease/pneumonitis** (see Warnings and precautions and Adverse drug reactions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) Tykerb should be discontinued in patients who experience pulmonary symptoms indicative of interstitial lung disease/pneumonitis which are NCI CTCAE grade 3 or higher. **Diarrhoea** (see Warnings and precautions and Adverse drug reactions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) Tykerb should be interrupted in patients with diarrhoea which is NCI CTCAE grade 3 or grade 1 or 2 with complicating features (moderate to severe abdominal cramping, nausea or vomiting greater than or equal to NCI CTCAE grade 2, decreased performance status, fever, sepsis, neutropenia, frank bleeding or dehydration). Tykerb may be reintroduced at a lower dose (reduced from 1250 mg/day to 1000 mg/day or from 1500 mg/day to 1250 mg/day) when diarrhoea resolves to grade 1 or less. Tykerb should be permanently discontinued in patients with NCI CTCAE grade 4 diarrhoea. **Severe Cutaneous Reactions** (see Warnings and precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) Tykerb should be discontinued in patients who experience severe progressive skin rash with blisters or mucosal lesions. **Other toxicities** Discontinuation or interruption of Tykerb may be considered if a patient develops toxicity greater than or equal to NCI CTCAE grade 2. Dosing can be restarted at the standard dose of 1250 mg/day or 1500 mg/day, if the toxicity improves to grade 1 or lower If the toxicity recurs, Tykerb should be restarted at a lower dose (reduced from 1250 mg/day to 1000 mg/day or from 1500 mg/day to 1250 mg/day). **Special population** **Renal impairment** There is no experience of Tykerb in patients with severe renal impairment, however patients with renal impairment are unlikely to require dose modification of Tykerb given that under 2% of an administered dose (lapatinib and metabolites) is eliminated renally (see Clinical pharmacology – Pharmacokinetics – Special populations – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic impairment** Tykerb is metabolized in the liver. Moderate and severe hepatic impairment have been associated with 56% and 85% increases in systemic exposure, respectively. Administration of Tykerb to patients with hepatic impairment requires caution due to increased exposure (see Warnings and precautions and Clinical pharmacology – Pharmacokinetics – Special populations – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients with severe hepatic impairment (Child-Pugh Class C) should have their Tykerb dose reduced. A dose reduction from 1250 mg/day to 750 mg/day or from 1500 mg/day to 1000 mg/day in patients with severe hepatic impairment is predicted to adjust the area under the curve (AUC) to the normal range. However, there is no clinical data with this dose adjustment in patients with severe hepatic impairment (see Warnings and precautions and Clinical pharmacology – Pharmacokinetics – Special populations – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Pediatric patients (below 18 years)** The safety and efficacy of Tykerb in pediatric patients has not been established. **Geriatric patients (65 years or above)** There are limited data on the use of Tykerb in patients aged 65 years and older. See Table 10. 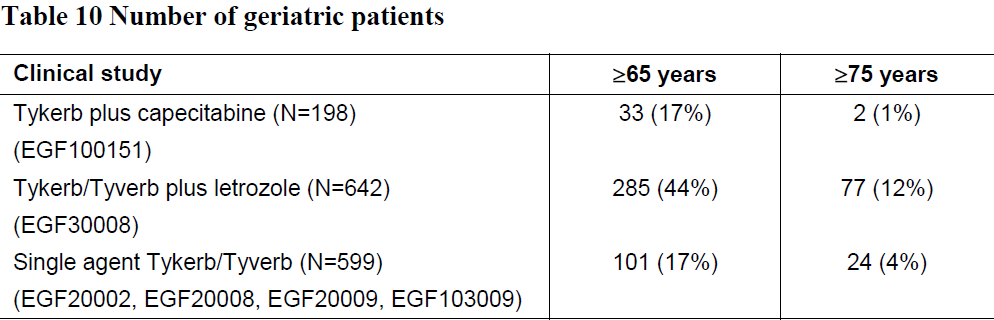 No age-based differences in the safety or efficacy of these regimens were observed. Other reported clinical experience has not identified differences in responses between the geriatric and younger patients. Greater sensitivity of geriatric patients cannot be ruled out.
ORAL
Medical Information
**Indications** Tykerb, in combination with capecitabine, is indicated for the treatment of patients with advanced/metastatic breast cancer whose tumours overexpress HER2 (ErbB2) and whose tumours have progressed after treatment with an anthracycline and, a taxane, and who have progressed on prior trastuzumab therapy in the metastatic setting. Tykerb, in combination with an aromatase inhibitor, is indicated for the treatment of postmenopausal women with hormone receptor-positive metastatic breast cancer whose tumours overexpress HER2/neu (ErbB2) and for whom endocrine therapy is indicated. The patients in the registration study were not previously treated with trastuzumab or an aromatase inhibitor (see Clinical studies – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). No data are available on the efficacy of this combination relative to trastuzumab in combination with an aromatase inhibitor chemotherapy in this patient population.
**Contraindications** Tykerb is contraindicated in patients with hypersensitivity to any of the ingredients (see Adverse drug reactions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
Manufacturer Information
NOVARTIS (SINGAPORE) PTE LTD
Glaxo Operations UK Ltd (trading as GlaxoWellcome Operations)
Glaxo SmithKline Australia Pty Ltd (Primary and Secondary Packager)
Glaxo Wellcome S.A. [Primary and secondary packager]
SANDOZ S.R.L
Active Ingredients
Documents
Package Inserts
Tykerb tablet 250mg PI.pdf
Approved: November 5, 2021
