Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**4.2 Posology and method of administration** Treatment should be initiated under supervision of a physician experienced in the treatment of the disease. When first starting treatment with Rebif, in order to allow tachyphylaxis to develop thus reducing adverse reactions it is recommended that patients be started at 8.8 micrograms dose subcutaneously and the dose be increased over a 4 week period to the targeted dose, according to the following schedule: 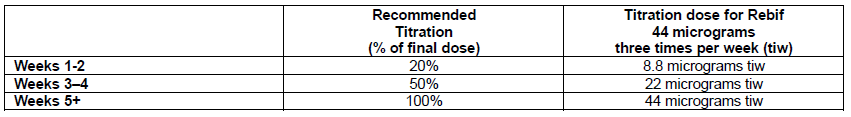 First demyelinating event The posology for patients who have experienced a first demyelinating event is 44 micrograms of Rebif given three times per week by subcutaneous injection. Relapsing multiple sclerosis The recommended posology of Rebif is 44 micrograms given three times per week by subcutaneous injection. A lower dose of 22 micrograms, also given three times per week by subcutaneous injection, is recommended for patients who cannot tolerate the higher dose in view of the treating specialist. Method of administration Rebif solution for injection in cartridge is intended for multidose use and should only be used with the RebiSmart autoinjector device following adequate training of the patient and/or carer. For administration, the instructions provided in the package leaflet and in the instruction manual provided with the RebiSmart autoinjector device should be followed. Prior to injection and for an additional 24 hours after each injection, an antipyretic analgesic is advised to decrease flu-like symptoms associated with Rebif administration. At the present time, it is not known for how long patients should be treated. Safety and efficacy with Rebif have not been demonstrated beyond 4 years of treatment. It is recommended that patients should be evaluated at least every second year in the 4-year period after initiation of treatment with Rebif and a decision for longer term treatment should then be made on an individual basis by the treating physician. Paediatric use No formal clinical trials or pharmacokinetic studies have been conducted in children or adolescents. However, limited published data suggest that the safety profile in adolescents from 12 to 16 years of age receiving Rebif 22 micrograms subcutaneously three times per week is similar to that seen in adults. There is very limited information on the use of Rebif in children under 12 years of age and therefore Rebif should not be used in this population.
SUBCUTANEOUS
Medical Information
**4.1 Therapeutic indications** Rebif is indicated for the treatment of - patients with a single demyelinating event with an active inflammatory process, if alternative diagnoses have been excluded, and if they are determined to be at high risk of developing relapsing multiple sclerosis (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - patients with relapsing multiple sclerosis. In clinical trials, this was characterised by two or more acute exacerbations in the previous two years (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Efficacy has not been demonstrated in patients with secondary progressive multiple sclerosis without ongoing relapse activity (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**4.3 Contraindications** - Hypersensitivity to natural or recombinant interferon-β, or to any excipients. - Current severe depression and/or suicidal ideation (see sections 4.4 and 4.8 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
L03AB07
interferon beta-1a
Manufacturer Information
MERCK PTE. LTD.
Merck Serono S.p.A (Bari)
Active Ingredients
Documents
Package Inserts
1.4.3 Proposed Rebif Cartridge 44mcg PI (clean).pdf
Approved: June 15, 2021
