Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Posology and method of administration** The optimum dosage of XANAX™ (alprazolam) should be individualized based upon the severity of the symptoms and individual patient response. In patients who require higher doses, dosage should be increased cautiously to avoid adverse effects. In general, patients who have not previously received psychotropic medications will require somewhat lower doses than those previously treated with minor tranquilizers, antidepressants, or hypnotics. It is recommended that the general principle of using the lowest effective dose be followed, especially in elderly or debilitated patients to preclude the development of oversedation or ataxia. If XANAX™ XR is to be given once daily, it is preferable to administer the dose in the morning. The tablets should be taken intact; they should not be chewed, crushed, or broken. Dosage recommendations for XANAX™ XR Tablets for use in panic disorders are based on short term clinical trials in patients with panic disorders and a comparable pharmacokinetic profile in normal subjects, between XANAX™ Tablets given three or four times daily and XANAX™ XR Tablets given twice daily. Dosing recommendations for XANAX™ XR Tablets for use in anxiety, depression, mixed anxiety-depression and in geriatric patients are based on a comparable pharmacokinetic profile in normal subjects between XANAX™ Tablets given three or four times daily and XANAX™ XR Tablets given twice daily. When equal daily doses of XANAX™ Tablets, dosed three or four times daily, and XANAX™ XR Tablets, dosed twice daily, were given, the steady-state serum concentration levels of alprazolam achieved with XANAX™ XR fell between the steady-state peak and trough serum concentration levels obtained with XANAX™ Tablets. The total amount of alprazolam absorbed is equivalent for XANAX™ Tablets and XANAX™ XR Tablets. 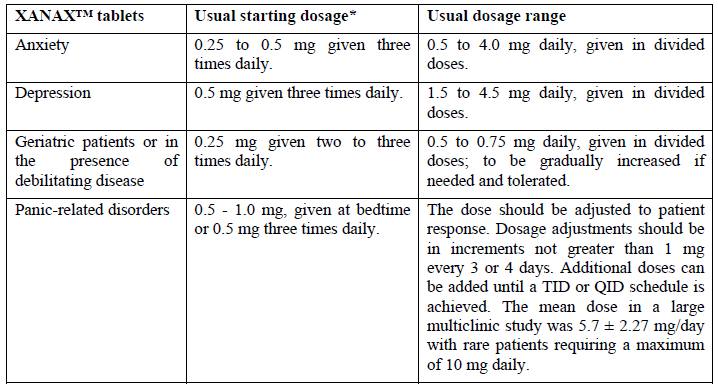 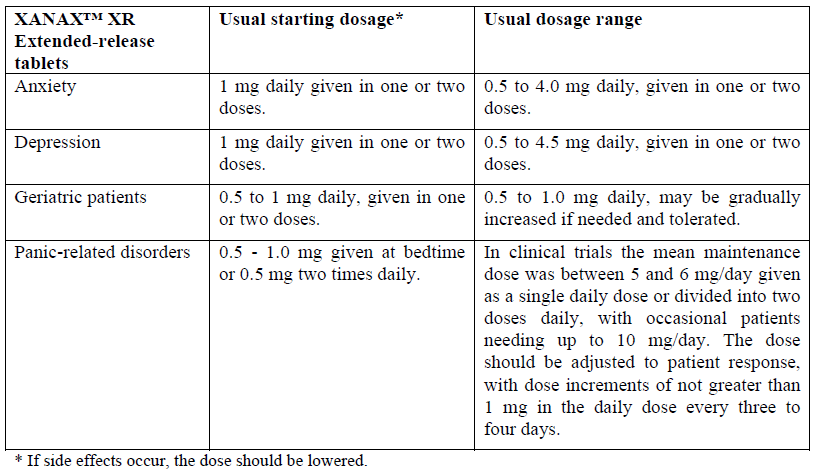 The safety and efficacy of XANAX™ in children less than 18 years of age has not been established. **Duration of Treatment** The risk of dependence may increase with dose and duration of treatment, therefore, the lowest possible effective dose and duration should be used and the need for continued treatment reassessed frequently (see section **4.4 Special warnings and precautions for use** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Discontinuation therapy** To discontinue alprazolam treatment, the dosage should be reduced slowly in keeping with good medical practice. It is suggested that the daily dosage of XANAX™ be decreased by not more than 0.5 mg every 3 days. Some patients may require an even slower dosage reduction (see section **4.4 Special warnings and precautions for use** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
ORAL
Medical Information
**4.1. Therapeutic indications** XANAX™ Tablets (alprazolam) are indicated for the treatment of: - **Anxiety states (anxiety neuroses)** Symptoms which occur in such patients include anxiety, tension, agitation, insomnia, apprehension, irritability and/or autonomic hyperactivity resulting in a variety of somatic complaints. - **Mixed anxiety-depression** Symptoms of both anxiety and depression occur simultaneously in such patients. - **Neurotic or reactive depression** Such patients primarily exhibit a depressed mood or a pervasive loss of interest or pleasure. Symptoms of anxiety, psychomotor agitation and insomnia are usually present. Other characteristics include appetite disturbances, changes in weight, somatic complaints, cognitive disturbances, decreased energy, feeling of worthlessness or guilt, or thoughts of death or suicide. - **Anxiety states, mixed anxiety-depression, or depression** associated with other diseases, such as the **chronic phase** of alcohol withdrawal and functional or organic disease, particularly certain gastrointestinal, cardiovascular, or dermatological disorders. - **Panic related disorders** XANAX™ is indicated in the treatment of panic disorder with or without some phobic avoidance. XANAX™ is also indicated for the blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. The effectiveness of XANAX™ in the treatment of anxiety, anxiety associated with depression and neurotic (reactive) depression for long-term use exceeding six months has not been established by systematic clinical trials; however, patients with panic-related disorders have been effectively treated for up to eight months. The physician should periodically reassess the usefulness of the drug for the individual patient.
**4.3. Contraindications** XANAX™ is contraindicated in patients with known hypersensitivity to the benzodiazepines, alprazolam, or to any component of these products’ formulations.
N05BA12
alprazolam
Manufacturer Information
VIATRIS PRIVATE LIMITED
SANICO NV
Active Ingredients
Documents
Package Inserts
XANAX TABLET PI.pdf
Approved: May 14, 2021
