Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**DOSAGE REGIMEN AND ADMINISTRATION** **Dosage regimen** **General target population** The recommended dose of Zykadia is 450 mg taken orally once daily with food at the same time each day. The maximum recommended dose is 450 mg taken orally once daily with food. Continue treatment as long as the patient is deriving clinical benefit from therapy. **Dose adjustments** Temporary dose interruption and/or dose reduction of Zykadia therapy may be required based on individual safety and tolerability. If dose reduction is required due to an adverse drug reaction not listed in Table 1, then the daily dose of Zykadia should be reduced by decrements of 150 mg. Early identification and management of adverse drug reactions with standard supportive care measures should be considered. Zykadia should be discontinued in patients unable to tolerate 150 mg taken once daily with food. Table 1 summarizes recommendations for dose interruption, reduction, or discontinuation of Zykadia in the management of selected adverse drug reactions (ADRs). 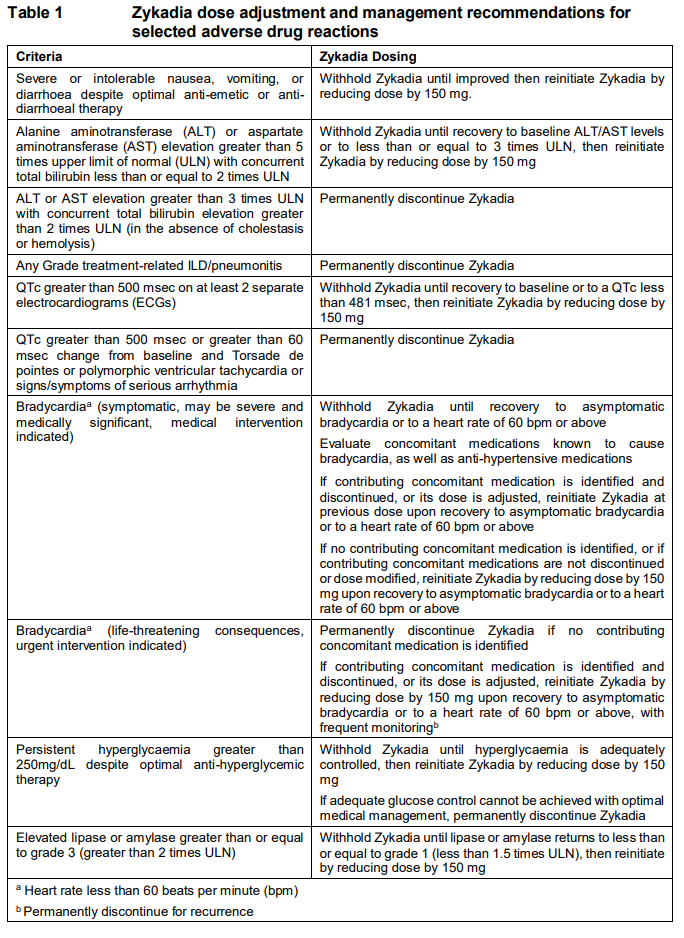 **Strong CYP3A inhibitors** Concurrent use of strong CYP3A inhibitors should be avoided (see section INTERACTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If concomitant use of a strong CYP3A inhibitor is unavoidable, the dose of Zykadia should be reduced by approximately one-third, rounded to the nearest multiple of the 150 mg dosage strength. After discontinuation of a strong CYP3A inhibitor, resume the Zykadia dose that was taken prior to initiating the strong CYP3A inhibitor. **Special populations** **Renal impairment** No dose adjustment is necessary in patients with mild to moderate renal impairment. Caution should be used in patients with severe renal impairment as there is no experience with Zykadia in this population (see section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic impairment** For patients with severe hepatic impairment (Child-Pugh C), the dose of Zykadia should be reduced by approximately one-third, rounded to the nearest multiple of the 150 mg dosage strength. No dose adjustment is necessary in patients with mild ((Child-Pugh) A) or moderate (Child-Pugh B) hepatic impairment (see section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Pediatric patients (below 18 years)** The safety and efficacy of Zykadia have not been established in pediatric patients. **Geriatric patients (65 years or above)** The limited data on the safety and efficacy of Zykadia in patients aged 65 years and older do not suggest that a dose adjustment is required in elderly patients (see section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Method of administration** Zykadia should be administered orally once daily with food at the same time every day. Food can range from a snack to a full meal (see section INTERACTIONS and section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Zykadia should be swallowed whole with water. Zykadia should not be chewed or crushed. If a dose is missed, the patient should make up that dose, unless the next dose is due within 12 hours. If vomiting occurs during the course of treatment, the patient should not take an additional dose, but should continue with the next scheduled dose.
ORAL
Medical Information
**INDICATIONS** Zykadia is indicated for the treatment of patients with anaplastic lymphoma kinase (ALK)- positive, locally advanced or metastatic non-small cell lung cancer (NSCLC), with or without metastases to the brain.
**CONTRAINDICATIONS** None.
Pending
xpending
Manufacturer Information
NOVARTIS (SINGAPORE) PTE LTD
Novartis Pharma Stein AG
Active Ingredients
Documents
Package Inserts
Zykadia capsule 150mg PI.pdf
Approved: June 30, 2021
