Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET
**6 Dosage & Administration** HIBRUKA should be administered under the direction of a physician specialized in the diagnosis and treatment of patients with the intended indications. HIBRUKA tablets should be taken orally once daily at approximately the same time each day, either before or after meal. Swallow the whole tablet with water. Do not break, crush or chew the tablets. The recommended dose of HIBRUKA is 150 mg (three 50 mg tablets) orally once daily until disease progression or unacceptable toxicity. **Missed dose** If a dose is missed at the scheduled time, it should be taken as soon as possible only if it is at least 8 hours before the next dose, and return to the normal schedule in the following day. Do not take extra tablets to make up for the missed dose. **Dosage Adjustments** Recommended dose modifications are provided in Table 1. 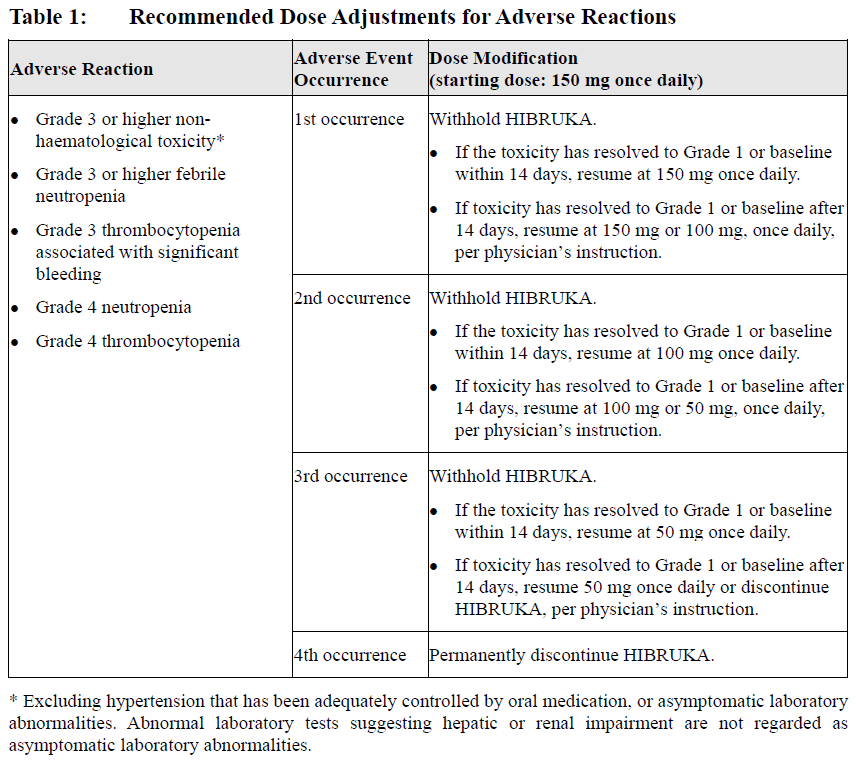 Asymptomatic lymphocytosis is usually not regarded as an adverse reaction. Patients who have experienced such event can continue HIBRUKA under the direction of the treating physician. **Co-administration with CYP3A4 Inhibitors or Inducers** No clinical studies of drug-drug interaction have been conducted. Caution should be taken when co-administered with CYP3A4 inhibitors or inducers. Co-administration with strong and moderate CYP3A4 inhibitors or inducers should be avoided. **Use in Specific Populations** Hepatic Impairment A pharmacokinetic study of orelabrutinib in patients with hepatic impairment has not been conducted. The use of orelabrutinib in patients with hepatic impairment is not recommended. Renal Impairment No dose modification is recommended in patients with mild renal impairment. Patients with moderate or severe renal impairment must use HIBRUKA with caution under the direction of a physician, and renal function should be closely monitored (See _9\. Warnings and Precautions_ and _17\. Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Geriatric Use No dose modification is required for elderly patients (See _12\. Geriatric Use_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Paediatric Use The safety and efficacy of HIBRUKA in paediatric patients have not been established.
ORAL
Medical Information
**4 Indications** HIBRUKA is indicated for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy.
**8 Contraindications** HIBRUKA is contraindicated in patients with: - Severe hepatic impairment; - Hypersensitivity (manifested by symptoms such as anaphylactic or anaphylactoid reaction) to HIBRUKA or to any of the excipients (see _18 List of Excipients_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
Pending
xpending
Manufacturer Information
FORTREA SINGAPORE PTE. LTD.
WuXi STA Pharmaceutical Co., Ltd.
Changzhou SynTheAll Pharmaceutical Co., Ltd. (ASD Manufacturing)
Active Ingredients
Documents
Package Inserts
Hibruka Tablet PI.pdf
Approved: June 19, 2023
