Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**Dosage and Administration** **Dosage** IMBRUVICA® should be administered orally once daily with a glass of water at approximately the same time each day. The capsules or tablets should be swallowed whole with water. Do not open, break, or chew the capsule. Do not break or chew the tablets. IMBRUVICA® must not be taken with grapefruit juice. IMBRUVICA® should continue until disease progression or no longer tolerated by the patient. _**Mantle Cell Lymphoma**_ The recommended dose of IMBRUVICA® for MCL is 560 mg once daily until disease progression or no longer tolerated by the patient. _**Chronic Lymphocytic Leukemia / Small Lymphocytic Lymphoma (CLL/SLL) and Waldenström’s macroglobulinemia (WM)**_ The recommended dose of IMBRUVICA® for CLL/SLL or WM is 420 mg once daily. For CLL/SLL, IMBRUVICA® can be administered until disease progression or is no longer tolerated by the patient as a single agent or in combination with anti-CD20 therapy (rituximab or obinutuzumab), or in combination with bendamustine and rituximab (BR). In combination with venetoclax, IMBRUVICA® should be administered as a single agent for 3 cycles (1 cycle is 28 days), followed by 12 cycles of IMBRUVICA® plus venetoclax. For WM, IMBRUVICA® can be administered until disease progression or no longer tolerated as a single agent or in combination with rituximab. For additional information concerning rituximab, BR, ventoclax or obinutuzumab see the corresponding local rituximab, bendamustine, venetoclax or obinutuzumab prescribing information. When administering IMBRUVICA® in combination with anti-CD20 therapy, it is recommended to administer IMBRUVICA® prior to anti-CD20 therapy when given on the same day. **Dose modification guidelines** The concomitant use of moderate and strong CYP3A inhibitors can increase the exposure of ibrutinib (see _Interactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Avoid co-administration with strong or moderate CYP3A inhibitors and consider alternative agents with less CYP3A inhibition. Concomitant use of strong CYP3A inhibitors which would be taken chronically (e.g. ketoconazole, indinavir, nelfinavir, ritonavir, saquinavir, clarithromycin, telithromycin, itraconazole, nefazodone, cobicistat) is not recommended. For short-term use (treatment for 7 days or less) of strong CYP3A inhibitors (e.g. antifungals and antibiotics), consider interrupting IMBRUVICA® therapy until the CYP3A inhibitor is no longer needed. See recommended dose modifications in the “interactions” section if a moderate CYP3A inhibitor must be used (e.g. fluconazole, erythromycin, amprenavir, aprepitant, atazanavir, ciprofloxacin, crizotinib, diltiazem, fosamprenavir, imatinib, verapamil, amiodarone, dronedarone) (see _Interactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients taking concomitant strong or moderate CYP3A inhibitors should be monitored more closely for signs of IMBRUVICA® toxicity. IMBRUVICA® therapy should be withheld for any new onset or worsening Grade 2 cardiac failure, Grade 3 cardiac arrhythmias, Grade ≥ 3 non-hematological toxicities, Grade 3 or greater neutropenia with infection or fever, or Grade 4 hematological toxicities. Once the symptoms of the toxicity have resolved to Grade 1 or baseline (recovery), resume IMBRUVICA® therapy at the recommended dose as per the tables below. Recommended dose modifications for non-cardiac events are described below: 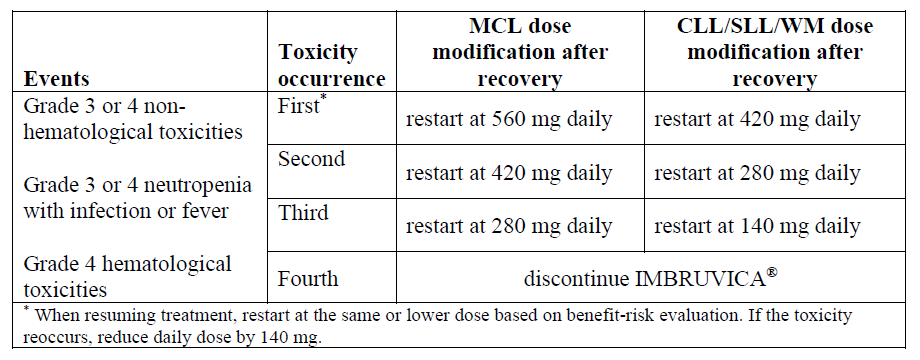 Recommended dose modifications for events of cardiac failure or cardiac arrhythmias are described below: 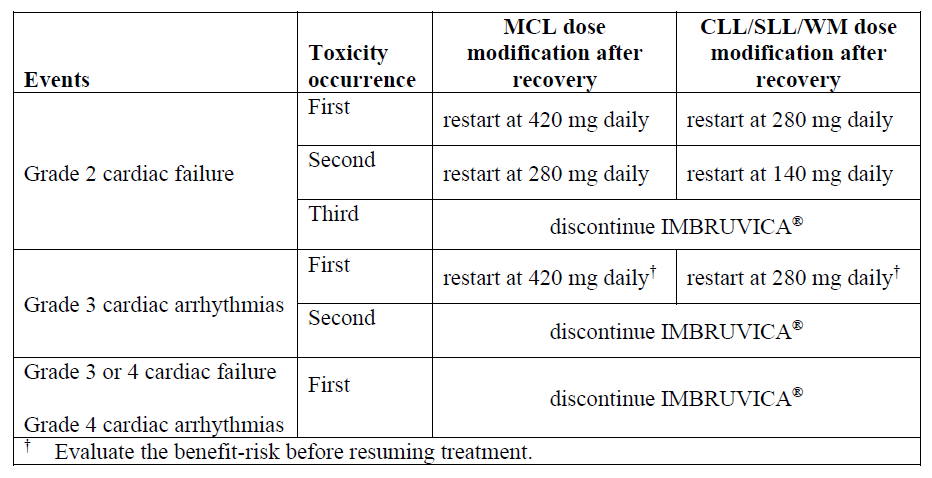 **Missed dose** If a dose of IMBRUVICA® is not taken at the scheduled time, it can be taken as soon as possible on the same day with a return to the normal schedule the following day. The patient should not take extra doses to make up the missed dose. **Special populations** _**Pediatrics (18 years of age and younger)**_ The safety and efficacy of IMBRUVICA® in children have not yet been evaluated. _**Renal impairment**_ Ibrutinib has minimal renal clearance. No specific clinical studies have been conducted in patients with renal impairment. Patients with mild or moderate renal impairment were treated in IMBRUVICA® clinical studies. No dose adjustment is needed for patients with mild or moderate renal impairment (greater than 30 mL/min creatinine clearance). Hydration should be maintained and serum creatinine levels monitored periodically. There are no data in patients with severe renal impairment or patients on dialysis (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Hepatic impairment**_ Ibrutinib is metabolized in the liver. In a hepatic impairment study, data showed an increase in ibrutinib exposure (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For patients with mild liver impairment (Child-Pugh classes A), the recommended dose is 140 mg daily. Monitor patients for signs of IMBRUVICA® toxicity and follow dose modification guidance as needed. It is not recommended to administer IMBRUVICA® to patients with moderate and severe hepatic impairment (Child-Pugh classes B and C).
ORAL
Medical Information
**Indications** **Mantle Cell Lymphoma (MCL)** IMBRUVICA® is indicated for the treatment of adult patients with MCL who have received at least one prior therapy. In the clinical study, efficacy was demonstrated based on overall response rate. Improvements in survival or disease-related symptoms have not been established. **Chronic lymphocytic leukemia / Small lymphocytic lymphoma (CLL/SLL)** IMBRUVICA® as a single agent, or in combination with rituximab or obinutuzumab or venetoclax, is indicated for the treatment of adult patients with previously untreated CLL/SLL. IMBRUVICA® as a single agent, or in combination with bendamustine and rituximab, is indicated for the treatment of patients with CLL/SLL who have received at least one prior therapy. **Waldenström’s macroglobulinemia (WM)** IMBRUVICA® as a single agent, or in combination with rituximab, is indicated for the treatment of patients with WM.
**Contraindications** IMBRUVICA® is contraindicated in patients who have known hypersensitivity (e.g., anaphylactic and anaphylactoid reactions) to ibrutinib or to the excipients in its formulation.
L01EL01
ibrutinib
Manufacturer Information
JOHNSON & JOHNSON INTERNATIONAL (SINGAPORE) PTE. LTD.
Cilag AG (Bulk Production and Primary Packager)
Janssen-Cilag SpA (Primary and Secondary Packager)
Active Ingredients
Documents
Package Inserts
Imbruvica PI.pdf
Approved: April 24, 2023
