Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**13 DOSAGE AND ADMINISTRATION** **13.1 Important Use Information** **DO NOT SUBSTITUTE** ONIVYDE pegylated liposomal for other drugs containing irinotecan HCl. **13.2 Recommended Use** Administer ONIVYDE pegylated liposomal prior to leucovorin and fluorouracil _\[see Clinical Studies (5)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. - The recommended dose of ONIVYDE pegylated liposomal is 70 mg/m2 administered by intravenous infusion over 90 minutes every 2 weeks. - A reduced starting dose of ONIVYDE pegylated liposomal of 50 mg/m2 should be considered in patients known to be homozygous for the UGT1A1\*28 allele. A dose increase of ONIVYDE pegylated liposomal to 70 mg/m2 should be considered if tolerated in subsequent cycles. - There is no recommended dose of ONIVYDE pegylated liposomal for patients with serum bilirubin above the upper limit of normal _\[see Adverse Reactions (9.1) and Clinical Studies (5)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Premedication Administer a corticosteroid and an anti-emetic 30 minutes prior to ONIVYDE pegylated liposomal infusion. **13.3 Dose Modifications for Adverse Reactions** All dose modifications should be based on the worst preceding toxicity. LV dose does not require adjustment. For Grade 1 and 2 toxicities, there are no dose modifications recommended. Dose adjustments, as summarized in Table 5 and Table 6, are recommended to manage Grade 3 or 4 toxicities related to ONIVYDE pegylated liposomal. For patients who start treatment with 50 mg/m2 ONIVYDE pegylated liposomal and do not dose escalate to 70 mg/m2, the recommended first dose reduction is to 43 mg/m2 and the second dose reduction is to 35 mg/m2. Patients who require further dose reduction should discontinue treatment. Patients who are known to be homozygous for UGT1A1\*28 and without drug related toxicities during the first cycle of therapy (reduced dose of 50 mg/m2) may have the dose of ONIVYDE pegylated liposomal increased to a total dose of 70 mg/m2 in subsequent cycles based on individual patient tolerance. 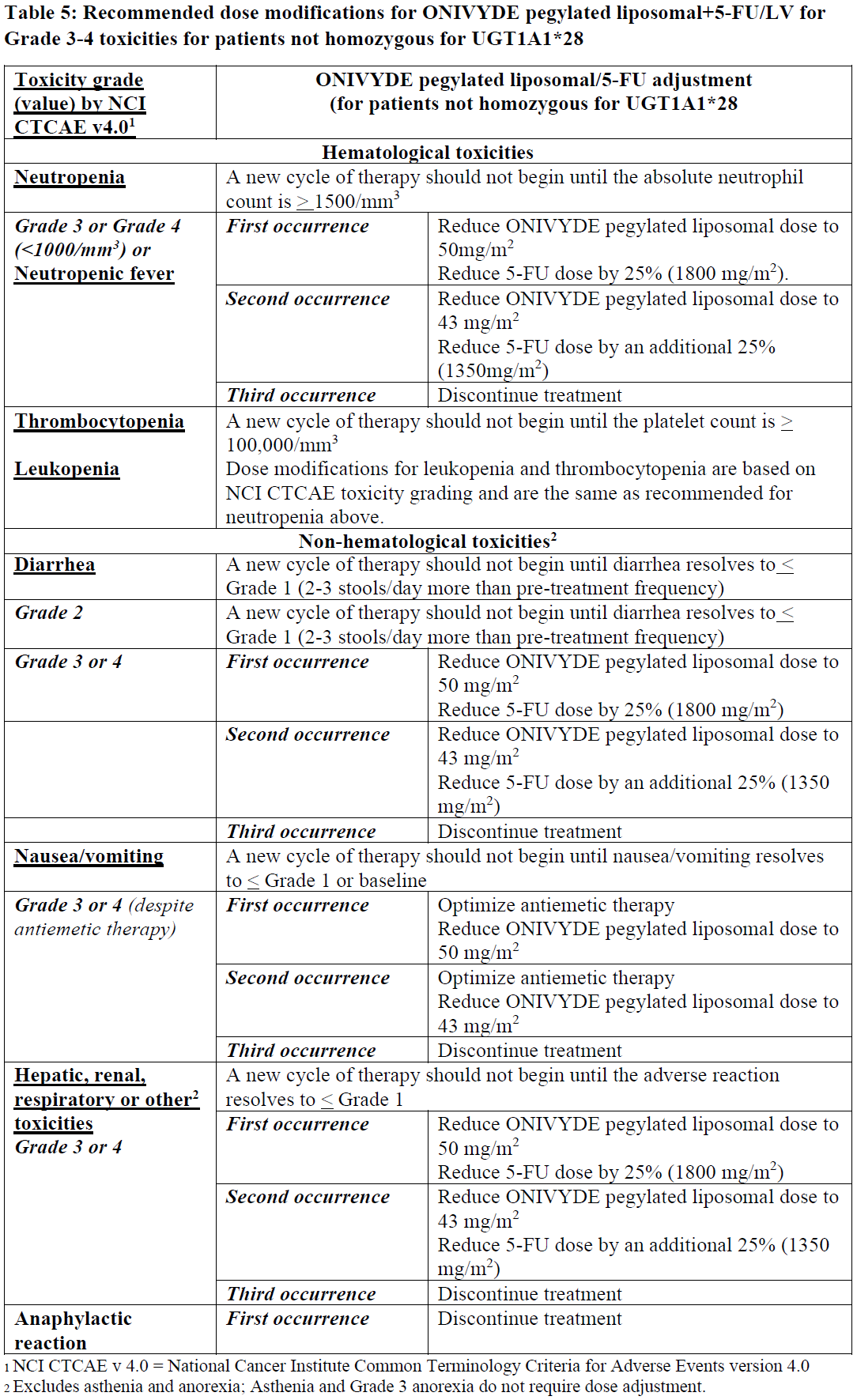 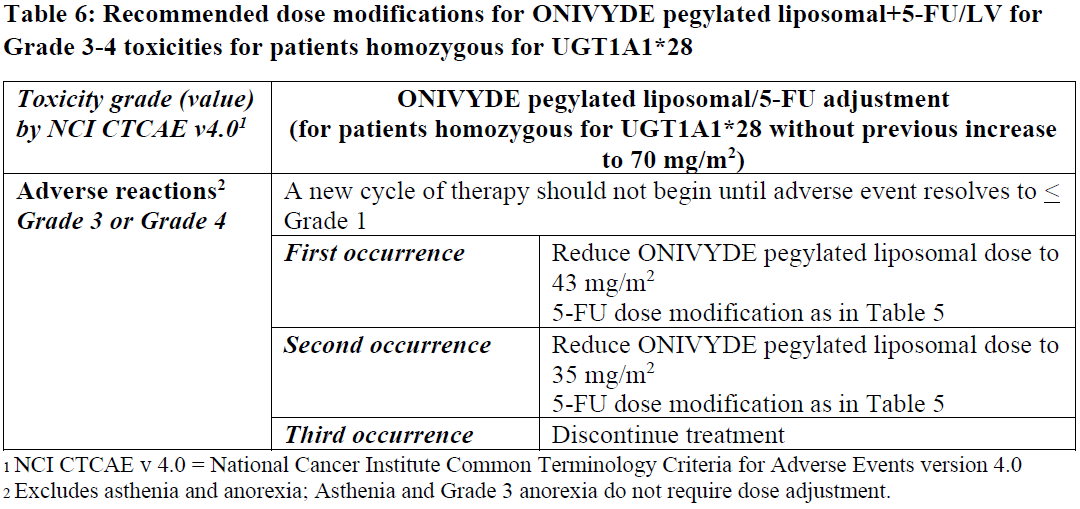 For recommended dose modifications of fluorouracil (5-FU) or leucovorin (LV), refer to the Full Prescribing Information; refer to Clinical Studies (5) – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. **13.4 Preparation and Administration** ONIVYDE pegylated liposomal is a cytotoxic drug. Follow applicable special handling and disposal procedures.1 Preparation - Withdraw the calculated volume of ONIVYDE pegylated liposomal from the vial using a needle not larger than 21 gauge. Dilute ONIVYDE pegylated liposomal in 500 mL 5% Dextrose Injection, USP or 0.9% Sodium Chloride Injection, USP and mix diluted dispersion by gentle inversion. - Protect diluted dispersion from light. - Chemical and physical stability for the diluted dispersion for infusion has been demonstrated at 15°C to 25°C for up to 4 hours or in the refrigerator (2°C to 8°C) for not more than 24 hours. Allow diluted dispersion to come to room temperature prior to administration. From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions are the responsibility of the user. - Do NOT freeze. Administration - Infuse diluted dispersion intravenously over 90 minutes. Do not use in-line filters. Discard unused portion. * * * **16 REFERENCES** 1. OSHA Hazardous Drugs. _OSHA._ http://www.osha.gov/SLTC/hazardousdrugs/index.html
INTRAVENOUS
Medical Information
**6 INDICATION AND USAGE** ONIVYDE pegylated liposomal is indicated, in combination with fluorouracil and leucovorin, for the treatment of patients with metastatic adenocarcinoma of the pancreas after disease progression following gemcitabine-based therapy. Limitation of Use: ONIVYDE pegylated liposomal is not indicated as a single agent for the treatment of patients with metastatic adenocarcinoma of the pancreas _\[see Clinical Studies (5)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_.
**7 CONTRAINDICATIONS** ONIVYDE pegylated liposomal is contraindicated in patients who have experienced a severe hypersensitivity reaction to ONIVYDE pegylated liposomal or irinotecan HCl.
L01XX19
xl 01 xx 19
Manufacturer Information
SERVIER (S) PTE LTD
Ipsen Biosciences, Inc.
Ajinomoto Althea, Inc.
IPSEN PHARMA BIOTECH
Active Ingredients
Documents
Package Inserts
Onivyde 4.3 mg per ml PI.pdf
Approved: December 7, 2022
