Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**DOSAGE AND ADMINISTRATION** _DO NOT INJECT INTRAVENOUSLY OR INTRADERMALLY._ HBvaxPRO (hepatitis B vaccine \[recombinant\], MSD), \[40 mcg/1.0 mL\] IS INTENDED ONLY FOR ADULT PREDIALYSIS/DIALYSIS PATIENTS. HBvaxPRO (hepatitis B vaccine \[recombinant\], MSD), \[5 mcg/0.5 mL and 10 mcg/1.0 mL ARE NOT INTENDED FOR USE IN PREDIALYSIS/DIALYSIS PATIENTS. This formulation is intended for single use only (no preservative). HBvaxPRO is for intramuscular injection. The deltoid muscle is the preferred site for intramuscular injection in adults. The anterolateral thigh is the recommended site for intramuscular injection in infants and young children. Data suggest that injections given in the buttocks are given frequently into fatty tissue instead of into muscle. Such injections have resulted in a lower seroconversion rate than is expected. HBvaxPRO may be administered subcutaneously to persons at risk of hemorrhage following intramuscular injections. However, when other aluminum-adsorbed vaccines have been administered subcutaneously, an increased incidence of local reactions including subcutaneous nodules has been observed. Therefore, subcutaneous administration should be used only in persons (e.g., hemophiliacs) at risk of hemorrhage following intramuscular injections. _Shake well before withdrawal and use._ Thorough agitation at the time of administration is necessary to maintain suspension of the vaccine. The vaccine should be used as supplied; no dilution or reconstitution is necessary. The full recommended dose of the vaccine should be used. NOTE: For All Formulations: Since none of the formulations contain a preservative, once the single-dose vial has been penetrated, the withdrawn vaccine should be used promptly, and the vial must be discarded. It is important to use a separate sterile syringe and needle for each individual patient to prevent transmission of hepatitis and other infectious agents from one person to another. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. After thorough agitation, HBvaxPRO is a slightly opaque, white suspension. _Three-Dose Regimen_ The vaccination regimen consists of three doses of vaccine given according to the following schedule: First injection: at elected date Second injection: ≥ 1 month after first injection Third injection: ≥ 1 month after second injection Within limits, the timing of successive injections may be adjusted to accommodate a variety of needs, such as coadministration with other EPI vaccines. For infants born of mothers who are HBsAg positive or mothers of unknown HBsAg status, treatment recommendations are described in the subsections titled: **Dosage Regimen for Infants Born to HBsAg-Positive Mothers and Dosage Regimen for Infants Born to Mothers of Unknown HBsAg Status.** A minimum of one month should separate successive injections of vaccine. Accelerated three-dose regimens (e.g. 0, 1, 2 months; 0, 2, 4 months) may induce protective antibody earlier in a slightly larger proportion of vaccinees. However, regimens that extend the time interval between the second and third injections (e.g. 0, 1, 6 months; 0, 1, 12 months) will ultimately seroconvert a similar proportion of vaccinees while inducing substantially higher antibody titers than accelerated regimens. _Two-Dose Regimen – Adolescents (11 – 15 years of age)_ An alternate two-dose regimen is available for routine vaccination of adolescents (11 to 15 years of age). The regimen consists of two doses of vaccine (10 mcg) given according to the following schedule: First injection: at elected date Second injection: 4–6 months later The dosing regimens of HBvaxPRO for specific populations other than predialysis/dialysis patients, regardless of the risk of infection with hepatitis B virus, are as follows: 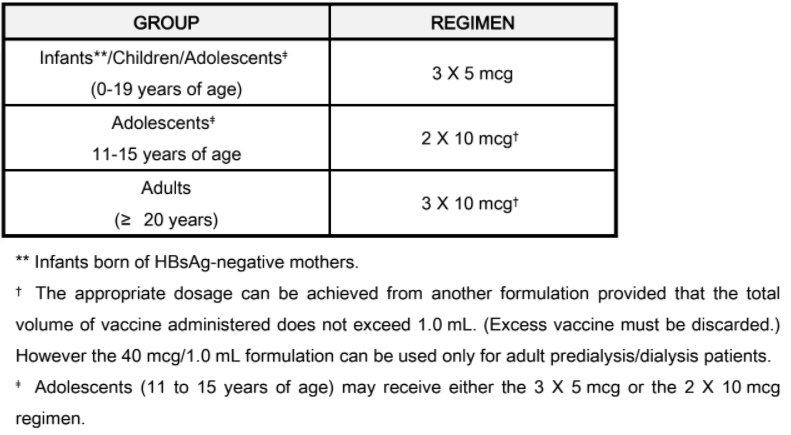 _**Dosage Regimen for Infants Born to HBsAg-Positive Mothers**_ Infants born to HBsAg-positive mothers are at high risk of becoming chronic carriers of hepatitis B virus and of developing the chronic sequelae of hepatitis B virus infection. Well-controlled studies have shown that administration of three 0.5 mL doses of hepatitis B immune globulin starting at birth is 75% effective in preventing establishment of the chronic carrier state in these infants during the first year of life. Protection is transient under these circumstances, and the effectiveness of the passively administered hepatitis B immune globulin declines thereafter. Results from clinical studies indicate that administration of one 0.5 mL dose of hepatitis B immune globulin at birth and three 5-mcg (0.5 mL) doses of HBvaxPRO, the first dose given within one week after birth, was 96% effective in preventing establishment of the chronic carrier state in infants born to HBsAg-positive and HBeAg-positive mothers. Testing for HBsAg and anti-HBs is recommended at 12–15 months to monitor the final success or failure of therapy. If HBsAg is not detectable, and anti-HBs is present, the child has been protected. The recommended dosage for infants born to HBsAg-positive mothers is as follows: 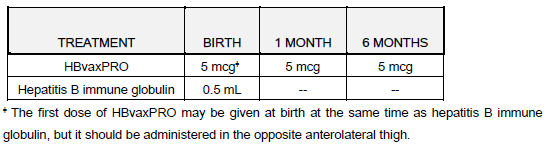 _**Dosage Regimen for Infants of Mothers of Unknown HBsAg Status**_ In the event that a mother’s HBsAg status is unknown, vaccination should be initiated as soon as possible with a 5 mcg dose of vaccine. If within 7 days of delivery, the mother is determined to be HBsAg-positive, the infant also should be given a dose of Hepatitis B Immune Globulin immediately; the vaccination series should then be completed with 5 mcg dosages. If the mother’s HBsAg antigen test is negative, then complete the vaccination series with the 5 mcg dosages. _**Predialysis/Dialysis Regimen**_ The recommended three-dose vaccination regimen for predialysis/dialysis patients is as follows:  A booster dose or revaccination with HBvaxPRO may be considered in predialysis/dialysis patients if the anti-HB level is less than 10 milli-international units/mL 1 to 2 months after the third dose. The need for booster doses of vaccine should be assessed by annual antibody testing, and a booster dose given when antibody levels decline to less than 10 milli-international units/mL. **Use with Other Vaccines** Results from clinical studies indicate that HBvaxPRO can be administered concomitantly with DTP (Diphtheria, Tetanus and whole cell Pertussis), OPV (oral Poliomyelitis vaccine), M-M-R II (Measles, Mumps, and Rubella Virus Vaccine Live), Liquid PedvaxHIB \[Haemophilus b Conjugate Vaccine (Meningococcal Protein Conjugate)\] or a booster dose of DTaP \[Diphtheria, Tetanus, acellular Pertussis\], using separate sites and syringes for injectable vaccines. No impairment of immune response to individually tested vaccine antigens was demonstrated. In addition, an HBsAg-containing product, COMVAX \[Haemophilus b Conjugate (Meningococcal Protein Conjugate) and Hepatitis B (Recombinant) Vaccine\], was given concomitantly with eIPV (enhanced inactivated Poliovirus vaccine) or VARIVAX \[Varicella Virus Vaccine Live (Oka/Merck)\], using separate sites and syringes for injectable vaccines. No impairment of immune response to these individually tested vaccine antigens was demonstrated. Revaccination of Nonresponders When persons who do not respond (anti-HBs < 10 international units/l) to the primary vaccine series are revaccinated, 15–25% produce an adequate antibody response after one additional dose and 30–50% after three additional doses. However, because data are insufficient concerning the safety of hepatitis B vaccine when additional doses in excess of the recommended two or three-dose series are administered, revaccination following completion of the primary series is not routinely recommended. Revaccination should only be considered for high-risk individuals, after weighing the benefits of vaccination against the potential risk of experiencing increased local or systemic adverse reactions. _**KNOWN or PRESUMED EXPOSURE TO HBsAg**_ There are no prospective studies directly testing the efficacy of a combination of hepatitis B immune globulin and HBvaxPRO in preventing clinical hepatitis B following percutaneous, ocular or mucous membrane exposure to hepatitis B virus. Since most persons with such exposures (e.g. health care workers) are candidates for the hepatitis B vaccine and since combined hepatitis B immune globulin plus vaccine is more efficacious than hepatitis B immune globulin alone in perinatal exposures, the following guidelines are recommended for persons who have been exposed to hepatitis B virus such as through (1) percutaneous (needlestick), ocular, mucous membrane exposure to blood known or presumed to contain HBsAg, (2) human bites by known or presumed HBsAg carriers, that penetrate the skin, or (3) following intimate sexual contact with known or presumed HBsAg carriers. Hepatitis B immune globulin (0.06 mL/kg) should be given as soon as possible after exposure and within 24 hours if possible. Hepatitis B vaccine, with the age-appropriate dose, (10 mcg for adults) should be given intramuscularly within 7 days of exposure and second and third doses given one and six months, respectively, after the first dose. _**Revaccination**_ The duration of the protective effect of HBvaxPRO in healthy vaccinees is unknown at present and the need for booster doses is not yet defined. _For Syringe Use Only:_ Withdraw the recommended dose from the vial using a sterile needle and syringe free of preservatives, antiseptics and detergents. _**Storage**_ Store vials at 2 – 8°C (35.6 – 46.4°F). _Do not freeze since freezing destroys potency_. Protect from light. When stored at 2 – 8°C, the pediatric, adult and dialysis formulations have a shelf life of 36 months. Storage above or below the recommended temperature may reduce potency. HBvaxPRO should be administered as soon as possible after being removed from refrigeration. HBvaxPRO can be administered provided total (cumulative multiple excursion) time out of refrigeration (at temperatures between 8°C and 25°C) does not exceed 72 hours. Cumulative multiple excursions between 0°C and 2°C are also permitted as long as the total time between 0°C and 2°C does not exceed 72 hours. These are not, however, recommendations for storage.
INTRAMUSCULAR
Medical Information
**INDICATIONS** All formulations of HBvaxPRO are indicated for immunization against infection caused by all known subtypes of hepatitis B virus. HBvaxPRO should also prevent hepatitis D (caused by the delta virus), since hepatitis D does not occur in the absence of hepatitis B infection.
**CONTRAINDICATIONS** Hypersensitivity to yeast or any other component of the vaccine.
J07BC01
hepatitis B, purified antigen
Manufacturer Information
MSD PHARMA (SINGAPORE) PTE. LTD.
Merck Sharp & Dohme LLC
Active Ingredients
Documents
Package Inserts
HBVaxpro Injection PI.pdf
Approved: September 20, 2022
