Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**DOSAGE AND ADMINISTRATION** Zoledronic Acid must not be mixed with calcium or other divalent cation-containing infusion solutions, such as Lactated Ringer’s solution, and should be administered as a single intravenous solution in a line separate from all other drugs. **Treatment of bone metastases and treatment of osteolytic lesions, in conjunction with standard antineoplastic therapy** _**Adults and elderly**_ The recommended dose in the treatment of bone metastases and treatment of osteolytic lesions is 4 mg zoledronic acid. The concentrate must be further diluted with 100 mL 0.9 % w/v sodium chloride or 5 % w/v glucose solution and given as an intravenous infusion lasting no less than 15 minutes every 3 to 4 weeks. Patients should also be administered an oral calcium supplement of 500 mg and 400 international units vitamin D daily. **Treatment of HCM** **_Adults and elderly_** The recommended dose in hypercalcaemia (albumin-corrected serum calcium ≥ 12.0 mg/dL or 3.0 mmol/L) is 4 mg zoledronic acid. The concentrate must be further diluted with 100 mL 0.9 % w/v sodium chloride or 5 % w/v glucose solution, given as a single intravenous infusion of no less than 15 minutes. Patients must be maintained well hydrated prior to and following administration of zoledronic acid. **Renal impairment** _**HCM:**_ Zoledronic Acid treatment in patients with hypercalcaemia of malignancy (HCM) and who have severe renal impairment should be considered only after evaluating the risks and benefit of treatment. In the clinical studies, patients with serum creatinine >400 micromol/L or >4.5 mg/dL were excluded. No dose adjustment is necessary in HCM patients with serum creatinine < 400 micromol/L or < 4.5 mg/dL. **Treatment of bone metastases and treatment of osteolytic lesions, in conjunction with standard antineoplastic therapy.** When initiating treatment with zoledronic acid in patients with multiple myeloma or metastatic bone lesions from solid tumours, serum creatinine levels and creatinine clearance (CrCl) should be determined. CrCl is calculated from serum creatinine levels using the Cockcroft-Gault formula. Zoledronic Acid is not recommended for patients presenting with severe renal impairment prior to initiation of therapy, which is defined for this population as CrCl < 30 mL/min. In clinical trials with zoledronic Acid, patients with serum creatinine > 265 micromol/L or > 3.0 mg/dL were excluded. In patients with bone metastases presenting with mild to moderate renal impairment prior to initiation of therapy, which is defined for this population as CrCl 30 to 60 mL/min, the following zoledronic acid dose is recommended. 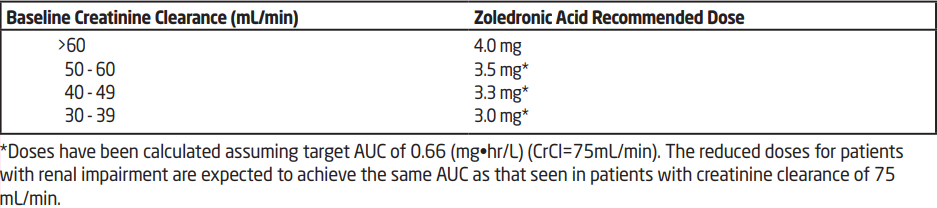 Following initiation of therapy, serum creatinine should be measured prior to each dose of zoledronic Acid and treatment should be withheld if renal function has deteriorated. In the clinical trials, renal deterioration was defined as follows: - For patients with normal baseline serum creatinine (< 1.4 mg/dL), an increase of ≥ 0.5 mg/dL; - For patients with an abnormal baseline creatinine (> 1.4 mg/dL), an increase of ≥ 1.0 mg/dL. In the clinical studies, zoledronic acid treatment was resumed only when the creatinine level returned to within 10% of the baseline value. Zoledronic acid should be resumed at the same dose as that prior to treatment interruption. **Instructions on preparing reduced doses of Zoledronic Acid** Withdraw an appropriate volume of the liquid concentrate needed, as follows: 4.4 mL for 3.5 mg dose 4.1 mL for 3.3 mg dose 3.8 mL for 3.0 mg dose For information on the reconstitution and dilution of Zoledronic acid, see section ‘instructions for use and handling’ and ‘information for healthcare professionals’ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. The withdrawn amount of liquid concentrate must be further diluted in 100 mL of sterile 0.9% w/v sodium chloride solution or 5% w/v glucose solution. The dose must be given as a single intravenous infusion of no less than 15 minutes.
INTRAVENOUS
Medical Information
**INDICATIONS** - Treatment of osteolytic, osteoblastic, and mixed bone metastases of solid tumours and osteolytic lesions of multiple myeloma, in conjunction with standard antineoplastic therapy. - Treatment of hypercalcaemia of malignancy (HCM).
**CONTRAINDICATIONS** Zoledronic acid concentrate is contraindicated in pregnancy, in breast-feeding women, patients with clinically significant hypersensitivity to zoledronic acid or other bisphosphonates or any of the excipients in the formulation of zoledronic acid.
M05BA08
zoledronic acid
Manufacturer Information
TEVA PHARMACEUTICAL INVESTMENTS SINGAPORE PTE. LTD.
Actavis Italy S.p.A.
PLIVA CROATIA Ltd
Active Ingredients
Documents
Package Inserts
Zoledronic acid 0.8mg-ml_PI.pdf
Approved: December 28, 2022
