Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**4.2 Posology and method of administration** Posology Fiasp® is a mealtime insulin for subcutaneous administration up to 2 minutes before the start of the meal, with the option to administer up to 20 minutes after starting the meal (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Dosing with Fiasp® is individual and determined in accordance with the needs of the patient. Fiasp® given by subcutaneous injection should be used in combination with intermediate-acting or long-acting insulin given at least once a day. In a basal-bolus treatment regimen approximately 50% of this requirement may be provided by Fiasp® and the remaining by intermediate-acting or long-acting insulin. The individual total daily insulin requirement in adults, adolescents and children may vary and is usually between 0.5 and 1 unit/kg/day. Blood glucose monitoring and insulin dose adjustment are recommended to achieve optimal glycaemic control. Adjustment of dose may be necessary if patients undertake increased physical activity, change their usual diet or during concomitant illness. Blood glucose levels should be monitored adequately under these conditions. The duration of action will vary according to the dose, injection site, blood flow, temperature and level of physical activity. Patients on basal-bolus treatment who forget a mealtime dose are advised to monitor their blood glucose level to decide if an insulin dose is needed. Patients should resume their usual dosing schedule at the next meal. The potency of insulin analogues, including Fiasp®, is expressed in units. One (1) unit of Fiasp® corresponds to 1 international unit of human insulin or 1 unit of other fast-acting insulin analogues. The early onset of action must be considered when prescribing Fiasp® (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Initiation_ _Patients with type 1 diabetes mellitus_ The recommended starting dose in insulin naïve patients with type 1 diabetes is approximately 50% of the total daily insulin dose and should be divided between the meals based on the size and composition of the meals. The remainder of the total daily insulin dose should be administered as intermediate-acting or long-acting insulin. As a general rule, 0.2 to 0.4 units of insulin per kilogram of body weight can be used to calculate the initial total daily insulin dose in insulin naïve patients with type 1 diabetes. _Patients with type 2 diabetes mellitus_ The suggested initial dose is 4 units at one or more meals. The number of injections and subsequent titration will depend on the individual glycaemic target and the size and composition of the meals. Dose adjustment may be considered daily based on self-measured plasma glucose (SMPG) on the previous day(s) according to Table 1. - Pre-breakfast dose should be adjusted according to the pre-lunch SMPG the previous day - Pre-lunch dose should be adjusted according to the pre-dinner SMPG the previous day - Pre-dinner dose should be adjusted according to the bedtime SMPG the previous day 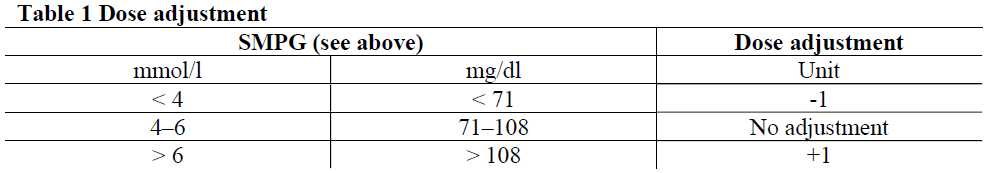 _Special populations_ _Elderly patients (≥ 65 years old)_ The safety and efficacy of Fiasp® have been established in elderly patients aged 65 to 75 years. Close glucose monitoring is recommended and the insulin dose should be adjusted on an individual basis (see sections 5.1 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The therapeutic experience in patients ≥ 75 years of age is limited. _Renal impairment_ Renal impairment may reduce the patient’s insulin requirements. In patients with renal impairment, glucose monitoring should be intensified and the dose adjusted on an individual basis (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Hepatic impairment_ Hepatic impairment may reduce the patient’s insulin requirements. In patients with hepatic impairment, glucose monitoring should be intensified and the dose adjusted on an individual basis (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Paediatric population_ Fiasp® can be used in adolescents and children from the age of 2 years (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). There is no clinical experience with the use of Fiasp® in children below the age of 2 years. Fiasp® is recommended to be administered prior to the meal (0–2 minutes), with the flexibility to administer up to 20 minutes after starting the meal in situations, when there is uncertainty about the meal intake. Transfer from other insulin medicinal products Close glucose monitoring is recommended during the transfer from other mealtime insulins and in the initial weeks thereafter. Converting from another mealtime insulin can be done on a unit-to-unit basis. Transferring a patient from another type, brand or manufacturer of insulin to Fiasp® must be done under strict medical supervision and may result in the need for a change in dose. Doses and timing of concurrent intermediate-acting or long-acting insulin medicinal products or other concomitant antidiabetic treatment may need to be adjusted. Method of administration _Subcutaneous injection_ Fiasp® is recommended to be administered subcutaneously by injection in the abdominal wall or the upper arm (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Injection sites should always be rotated within the same region in order to reduce the risk of lipodystrophy and cutaneous amyloidosis (see sections 4.4 and 4.8 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Administration with a syringe_ The vial is to be used with insulin syringes with the corresponding unit scale (units-100 or 100 units/ml). _Continuous subcutaneous insulin infusion (CSII)_ Fiasp® solution for injection in vial can be used for CSII in pumps suitable for insulin infusion and will cover both the bolus insulin requirement (approximately 50%) and basal insulin. It can be administered in accordance with the instructions provided by the pump manufacturer, preferably in the abdomen. When used with an insulin infusion pump, it should not be diluted or mixed with any other insulin medicinal products. Patients using CSII should be instructed in the use of the pump and use the correct reservoir and tubing for the pump (see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The infusion set (tubing and cannula) should be changed in accordance with the instructions in the product information supplied with the infusion set. Patients administering Fiasp® by CSII must be trained to administer insulin by injection and have alternate insulin therapy available in case of pump failure. _Intravenous use_ If necessary, Fiasp® can be administered intravenously by health care professionals. For intravenous use, it should be used at concentrations from 0.5 unit/ml to 1 unit/ml insulin aspart in infusion systems – using polypropylene infusion bags. Fiasp® must not be mixed with any other insulin or any other medicinal product except those mentioned in section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. For instructions on dilution of the medicinal product before administration, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Monitoring of blood glucose is necessary during insulin infusion. Care should be taken to ensure that the insulin is injected into the infusion bag and not simply the entry port.
INTRAVENOUS, SUBCUTANEOUS
Medical Information
**4.1 Therapeutic indications** Treatment of diabetes mellitus in adults, adolescents and children aged 2 years and above.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
A10AB05
insulin aspart
Manufacturer Information
NOVO NORDISK PHARMA (SINGAPORE) PTE LTD
Novo Nordisk A/S(Bagsvaerd)(Bulk Production and Primary Packaging)
Novo Nordisk Pharmaceutical Industries, LP (Bulk Production and Secondary packaging)
Active Ingredients
Documents
Package Inserts
FIASP SOLUTION FOR INJECTION IN VIAL PI.pdf
Approved: January 4, 2023
