Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**Dosage and Administration** **Dosage – Adults 18 years of age and older** The recommended dose of INVOKANA™ is 100 mg or 300 mg once daily. See Table 1 for dosage recommendations based on estimated glomerular filtration rate (eGFR). The 300 mg dose may be considered for patients with an eGFR ≥ 60 mL/min/1.73 m2 \[CrCl ≥ 60 mL/min\], who need tighter glycemic control and who have a low risk of adverse reactions associated with reduced intravascular volume with INVOKANA™ treatment (see _below_ and _Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). A starting dose of 100 mg once daily should be used in patients on loop diuretics and patients ≥ 75 years of age. In patients with evidence of reduced intravascular volume, correcting this condition prior to initiation of INVOKANA™ is recommended. For those patients who are tolerating INVOKANA™ 100 mg and who need tighter glycemic control, the dose can be increased to INVOKANA™ 300 mg (see _Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). When INVOKANA™ is used as add-on therapy with insulin or an insulin secretagogue (e.g., sulfonylurea), a lower dose of insulin or the insulin secretagogue may be considered to reduce the risk of hypoglycemia (see _Warnings and Precautions_ and _Adverse Reactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 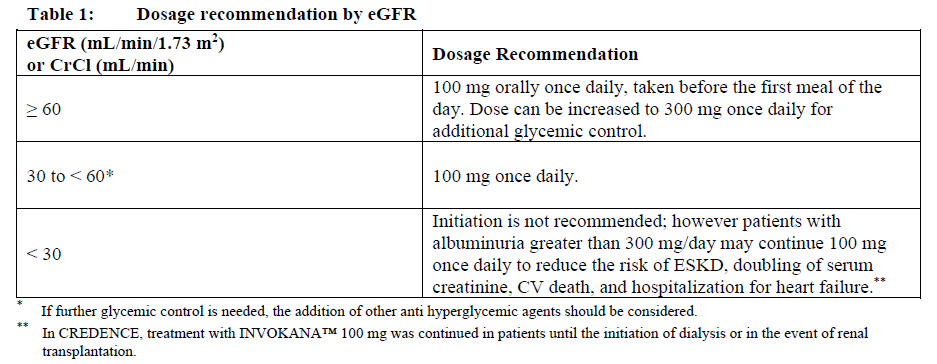 **Administration** INVOKANA™ should be taken orally once a day, preferably before the first meal of the day (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Tablets are to be swallowed whole. **Missed dose** If a dose is missed, it should be taken as soon as the patient remembers; however, a double dose should not be taken on the same day. **Special populations** **Pediatrics (< 18 years of age)** The safety and efficacy of INVOKANA™ have not been established in pediatric patients. **Elderly** In patients ≥ 75 years of age, the starting dose of INVOKANA™ is 100 mg once daily. Renal function and risk of volume depletion should be taken into account (see _Warnings and Precautions_ and _Adverse Reactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
ORAL
Medical Information
**Indications** **Monotherapy and Combination Therapy** INVOKANA™ is indicated as an adjunct to diet and exercise and standard care therapy: - to improve glycemic control in adults with type 2 diabetes mellitus. - to reduce the risk of major adverse cardiovascular events (cardiovascular death, nonfatal myocardial infarction and nonfatal stroke) in adults with type 2 diabetes mellitus and established cardiovascular disease (CVD) who have inadequate glycemic control. - to reduce the risk of end-stage kidney disease (ESKD), doubling of serum creatinine, cardiovascular (CV) death, and hospitalization for heart failure in adult patients with type 2 diabetes mellitus and diabetic nephropathy with albuminuria >300mg/day.
**Contraindications** History of a serious hypersensitivity reaction to INVOKANA™. Patients on dialysis. Hypersensitivity to the active substance or to any of the excipients (see _Adverse Reactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
A10BK02
canagliflozin
Manufacturer Information
JOHNSON & JOHNSON INTERNATIONAL (SINGAPORE) PTE. LTD.
Janssen Ortho, LLC
Janssen Cilag SpA
Active Ingredients
Documents
Package Inserts
Invokana PI.pdf
Approved: April 11, 2023
