Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**Dosage and Administration** **Dosage – Adults (≥18 years)** **_Recommended dose_** The recommended starting dose of BALVERSA™ is 8 mg orally once daily; with pharmacodynamically guided up-titration, based on serum phosphate concentrations and tolerability at 14 to 21 days, to 9 mg daily if criteria are met (see _Dosage and Administration - Dose Modifications_). _**Administration**_ Before taking BALVERSA™, patients must have confirmation of susceptible FGFR3 gene alterations as confirmed by a validated test (see _Pharmacodynamic Effects - Clinical studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The tablets should be swallowed whole with or without food. If vomiting occurs any time after taking BALVERSA™, the next dose should be taken the next day. Treatment should continue until disease progression or unacceptable toxicity occurs. _**Missed dose**_ If a dose of BALVERSA™ is missed, it can be taken as soon as possible. Resume the regular daily dose schedule for BALVERSA™ the next day. Extra tablets should not be taken to make up for the missed dose. _**Dose modifications**_ Pharmacodynamically-guided up-titration based on serum phosphate concentrations Serum phosphate (PO4) concentrations should be assessed between 14 and 21 days after initiating treatment. Up-titrate the dose to 9 mg daily as soon as possible if that serum phosphate (PO4) concentration is <5.5 mg/dL, and there is no drug-related toxicity. Dose reduction For possible dose reductions and management of adverse reactions see Tables 1 to 4. 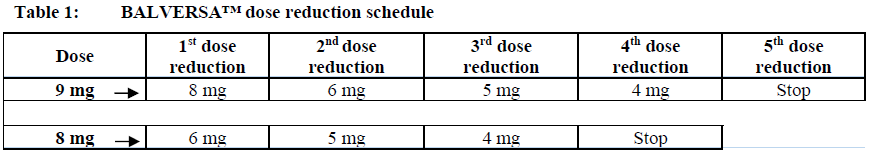 Hyperphosphatemia is an expected, transient laboratory abnormality of FGFR inhibitors (see _Pharmacodynamic Effects_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Phosphate concentrations should be monitored monthly. For elevated phosphate concentrations in patients treated with BALVERSA™ follow dose modification guidelines in Table 2. For all patients, phosphate intake should be restricted to 600–800 mg daily. For elevated phosphate concentrations (≥7.0 mg/dL) in patients treated with BALVERSA™, follow the dose modification guidelines in Table 2, and addition of a non-calcium containing phosphate binder (e.g., sevelamer carbonate) should be considered. 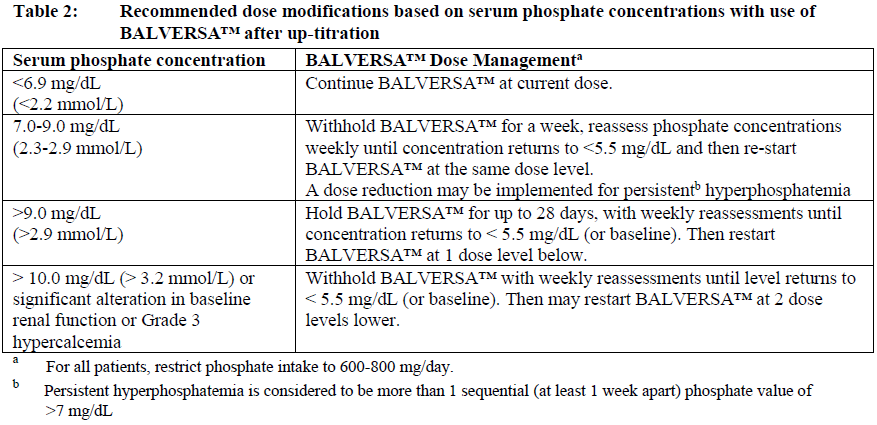 Eye disorder management Prior to initiating BALVERSA™, perform a baseline ophthalmological exam including an Amsler grid test, fundoscopy, visual acuity and, if available, an optical coherence tomography (OCT). To prevent and treat dry eyes, use artificial tear substitutes, hydrating or lubricating eye gels or ointments frequently, at least every 2 hours during waking hours. Severe treatment-related dry eye should be evaluated by an ophthalmologist. Subsequently examine patients monthly, including an Amsler grid test, and if any abnormality is observed, follow the management guidelines in Table 3. 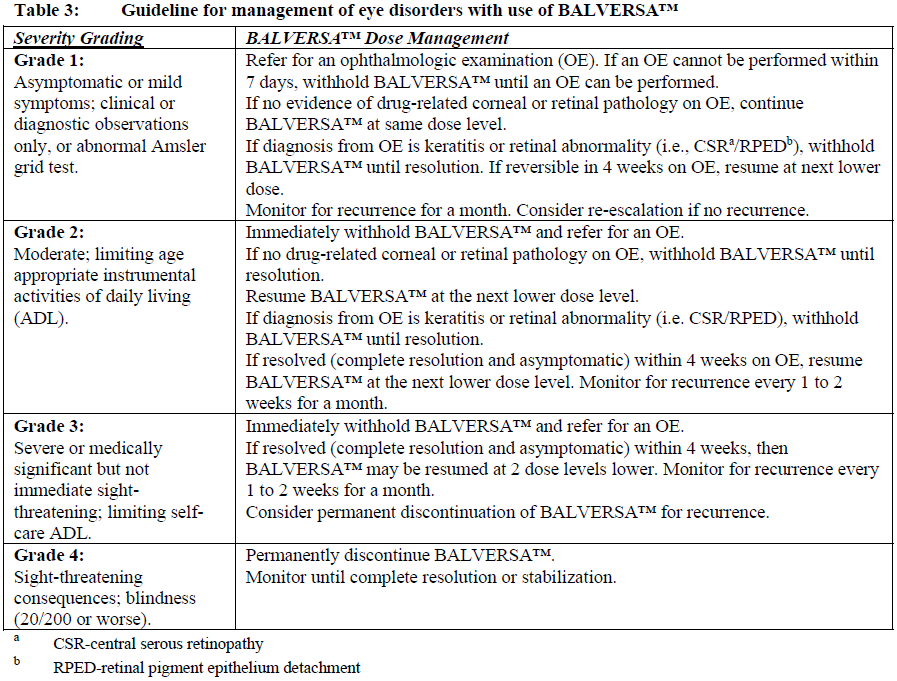 Dose modification for other adverse reactions Skin, mucosal, and nail changes have been observed with BALVERSA™. Follow dose modification guidelines in Table 4. 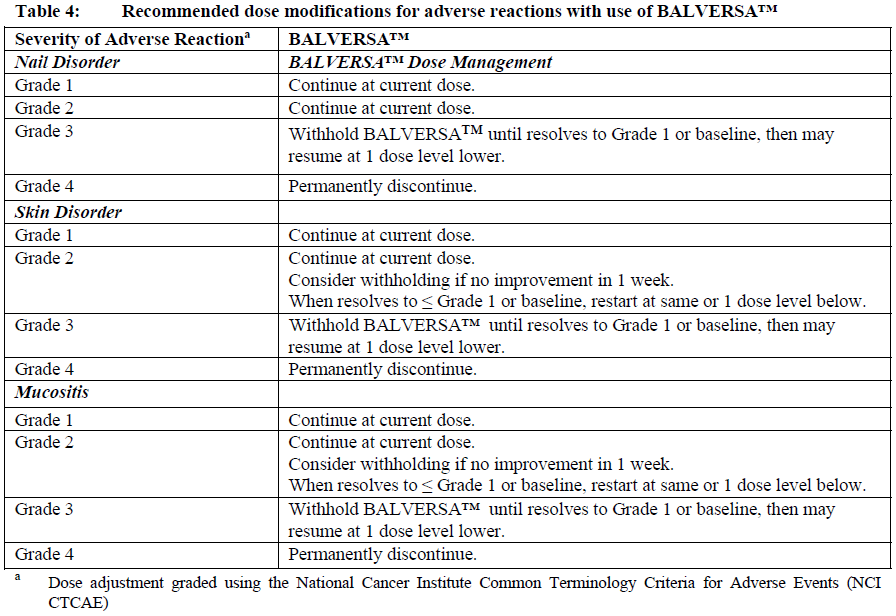 **Special populations** _**Pediatrics (17 years of age and younger)**_ The safety and efficacy of erdafitinib in children have not been established. No data are available. **_Elderly (65 years of age and older)_** Of the 416 patients treated with BALVERSA™ in clinical studies, 45% were 65 years of age or older, and 12% were 75 years of age or older. No overall differences in safety and effectiveness were observed between elderly and younger patients. No specific dose adjustments are considered necessary for elderly patients (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Renal impairment**_ Based on population pharmacokinetic (PK) analyses, no dose adjustment is required for patients with mild or moderate renal impairment (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). No data are available in patients with severe renal impairment. **_Hepatic impairment_** No dose adjustment is required for patients with mild or moderate hepatic impairment (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Limited data are available in patients with severe hepatic impairment.
ORAL
Medical Information
**Indications** BALVERSA™ is indicated for the treatment of adult patients with locally advanced or metastatic urothelial carcinoma (UC), whose tumors have susceptible fibroblast growth factor receptor (FGFR) 3 genetic alterations, who have disease progression during or following at least one line of prior chemotherapy including within 12 months of neoadjuvant or adjuvant chemotherapy (see _Pharmacodynamic Effects - Clinical Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**Contraindications** None.
Pending
xpending
Manufacturer Information
JOHNSON & JOHNSON INTERNATIONAL (SINGAPORE) PTE. LTD.
Janssen Cilag SpA
Active Ingredients
Documents
Package Inserts
Balversa Film Coated Tablets PI.pdf
Approved: March 8, 2022
