Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**Dosage** Dosage, mode, and frequency of administration depend on the severity of the infection, susceptibility of the pathogen, and condition of the patient. Cytaxin is an injectable antibiotic administered by the IM or IV route (given by slow injection or infusion). **Dosage in infants and children up to 12 years old,** unless otherwise prescribed, are given daily doses of 50 – 100 mg/kg body weight, divided into equal doses at intervals of 12–6 hours. In individual cases, patients with life-threatening infections were treated with daily amounts of 150 – 200 mg/kg body weight: these doses were well tolerated. Since renal clearance is not yet fully developed in premature infants, daily doses of 50 mg/kg body weight should not be exceeded. For the perioperative prophylaxis of infections, one of the above single doses is administered 30 – 60 minutes before the start of the surgery. Depending on the risk of infection, the same dose may be repeated. 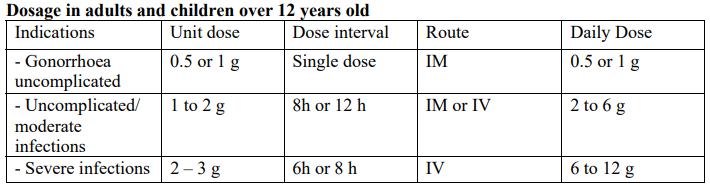 Where infection is caused by strains that are not usually sensitive, antibiotic sensitivity testing is the only means of confirming the efficacy of cefotaxime. For perioperative prophylaxis of infections, the administration of 1 – 2g Cefotaxime 30 – 60 minutes before the start of surgery is recommended. Depending on the risk of infection, the same dose may be repeated. In patients with a creatinine clearance less than 10 ml/minute, after an initial normal dose, the maintenance doses have to be reduced to one half of the normal dose, without change of the dose interval **Dosage in patients with impaired renal function** _Haemodialysis_: 1 to 2 g daily, depending on the severity of the infection; on dialysis days, Cefotaxime must be given after dialysis. In patients undergoing peritoneal dialysis: 1 to 2 g daily, depending on the severity of the infection; cefotaxime is not removed by peritoneal dialysis. _**Duration of treatment:**_ The duration of treatment depends on the patient’s response. It should be continued for at least three days after the body temperature has returned to normal. **Administration** _Intravenous injection_: For intravenous injection, the contents of one vial of Cytaxin 500mg are dissolved in at least 2 ml water for injections: Cytaxin 1 g are dissolved in at least 4 ml water for injections and Cefotaxime 2 g are dissolved in at least 10 ml water for injection and best use immediately. The solution is then injected over a period of 3 to 5 minutes. During post-marketing surveillance, potentially life-threatening arrhythmia has been reported in a very few patients who received rapid intravenous administration of cefotaxime through a central venous catheter. _Intravenous infusion_: If higher doses are required, they may be administered by intravenous infusion: For short infusion, 2 g Cefotaxime are dissolved in 40 ml water for injections or one of the usual infusion solutions (0.9% Sodium Chloride Injection, 5% Dextrose Injection, 5% Dextrose and 0.9% Sodium Chloride injection, Lactated Ringer's Injection) and then administered over approximately 20 minutes. For continuous drip infusion, 2 g Cefotaxime are dissolved in 100 ml of one of the above-mentioned infusion solutions and then administered over 50 – 60 minutes. Cefotaxime should not be mixed with other antibiotics in the same syringe or with other infusion solution; this applies above all to aminoglycosides. Sodium bicarbonate solutions must not be mixed with Cefotaxime. _Intramuscular administration_: The contents of one vial of Cytaxin 1 g are dissolved in 4 ml water for injections; the solution is then injected deep into the gluteus muscle. It is advisable not to inject more than 4 ml into either side. Intravenous injection is recommended if the daily dose exceeds 2 g or if Cefotaxime 1 g is administer more than twice daily. Pain resulting from the i.m. injection can be prevented by dissolving Cytaxin 1 g in the corresponding amount of 1% lidocaine solution, but in this case intravascular injection must be strictly avoided. The solution should be used immediately after reconstitution. Aseptic handling is particularly important if the solution is not intended for immediate use. 1. _For intramuscular and intravenous injection_ Cefotaxime sterile powder after reconstitution in water for injection is chemically stable: - up to 12 hours at room temperature (not exceeding 25°C) - up to 12 hours under refrigerated conditions (5 °C ± 3 °C) 2. _For infusion in infusion fluids_ Cefotaxime sterile powder is chemically stable at room temperature (not exceeding 25°C) and at refrigerated conditions (5 °C ± 3 °C) - up to 12 hours after reconstitution in the usual infusion solution (0.9% Sodium Chloride Injection, 5% Dextrose Injection, 5% Dextrose and 0.9% Sodium Chloride injection, Lactated Ringer's Injection).
INTRAVENOUS, INTRAMUSCULAR
Medical Information
**Indications** Severe infections caused by cefotaxime-susceptible pathogens; Infections - of the respiratory tract, including nose and throat - of the ear - of the kidneys and urinary tract, - of the skin and soft tissues - of the bones and joints, - of the genital organs including gonorrhoea, - of the abdominal region Sepsis, endocartitis, meningitis: for perioperative prophylaxis in patients who are at increased risk from infection, and for the prophylaxis of infections in patients with reduced resistance. Cefotaxime is generally effective against the following pathogens: Staphylococci, aerobic and anaerobic streptococci, Streptococcus pneumonia, Neisseria spp., Haemophilus influenzae, Eschericha coli, Citrobacter spp., Salmonella spp., Klebsiella spp., Enterobacter areogenes, Serratia spp., indole-positive and indole-negative Proteus spp., Yersinia entercolitica, Clostridium spp., and Bacteroides spp. Pathogens with varying susceptibility are: Streptococcus faecalis, Enterobacter colacae, Pseudomonas aeruginosa, and Bacteroides fragilis. There are not yet sufficient clinical experience with Salmonella typhi and parathyphi A and B infections. Cefotaxime is not effective against Treponema pallidum and Clostridium difficile. Combination therapy: In severe, life-threatening infections, the combination of Cefotaxime with aminoglycosides is indicated without awaiting the results of sensitivity tests. The two preparations must be administered separately. Infections with Pseudomonas aeruginosa may require concomitant treatment with other antibiotics effective against Pseudomonas.
**Contraindications** - Hypersensitivity to cephalosporins For pharmaceutical forms containing lidocaine: - Known history of hypersensitivity to lidocaine or other local anesthetics of the amide type - Non-paced heart block - Severe heart failure - Administration by the intravenous route - Infants aged less than 30 months of age
J01DD01
cefotaxime
Manufacturer Information
PHARMAKOE PTE. LTD.
TENAMYD PHARMACEUTICAL CORPORATION
Active Ingredients
Documents
Package Inserts
Cytaxin Powder for Solution for Injection PI.pdf
Approved: November 10, 2021
