Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**4.2 Posology and method of administration** ADCETRIS should be administered under the supervision of a physician experienced in the use of anti-cancer agents. Posology _Previously Untreated HL_ The recommended dose in combination with chemotherapy (doxorubicin \[A\], vinblastine \[V\] and dacarbazine \[D\] \[AVD\]) is 1.2 mg/kg administered as an intravenous infusion over 30 minutes on days 1 and 15 of each 28-day cycle for 6 cycles (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Primary prophylaxis with growth factor support (G-CSF) is recommended for all patients beginning with the first dose (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Refer to the product insert of chemotherapy agents given in combination with ADCETRIS for frontline treatment of patients with HL. _HL at increased risk of relapse or progression following ASCT_ The recommended dose is 1.8 mg/kg administered as an intravenous infusion over 30 minutes every 3 weeks. ADCETRIS treatment should start following recovery from ASCT based on clinical judgment. These patients should receive up to 16 cycles (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Relapsed or refractory HL_ The recommended dose is 1.8 mg/kg administered as an intravenous infusion over 30 minutes every 3 weeks. The recommended starting dose for the retreatment of patients with relapsed or refractory HL who have previously responded to treatment with ADCETRIS is 1.8 mg/kg administered as an intravenous infusion over 30 minutes every 3 weeks. Alternatively, treatment may be started at the last tolerated dose (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Treatment should be continued until disease progression or unacceptable toxicity (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients who achieve stable disease or better should receive a minimum of 8 cycles and up to a maximum of 16 cycles (approximately 1 year) (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Frontline PTCL_ The recommended dose in combination with chemotherapy (cyclophosphamide \[C\], doxorubicin \[H\], and prednisone \[P\]; \[CHP\]) is 1.8 mg/kg administered as an intravenous infusion over 30 minutes every 3 weeks for 6 to 8 cycles (See section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Primary prophylaxis with growth factor support (G-CSF) is recommended for all patients beginning with the first dose (See section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Refer to the product information of chemotherapy agents given in combination with ADCETRIS for treatment of patients with previously untreated PTCL. _Relapsed or refractory sALCL_ The recommended dose is 1.8 mg/kg administered as an intravenous infusion over 30 minutes every 3 weeks. The recommended starting dose for the retreatment of patients who have previously responded to treatment with ADCETRIS is 1.8 mg/kg administered as an intravenous infusion over 30 minutes every 3 weeks. Alternatively, treatment may be started at the last tolerated dose (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Treatment should be continued until disease progression or unacceptable toxicity (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients who achieve stable disease or better should receive a minimum of 8 cycles and up to a maximum of 16 cycles (approximately 1 year) (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _CTCL_ The recommended dose is 1.8 mg/kg administered as an intravenous infusion over 30 minutes every 3 weeks. Patients with CTCL should receive up to 16 cycles. _General_ Do not administer as an IV push or bolus. If the patient’s weight is more than 100 kg, the dose calculation should use 100 kg (see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Complete blood counts should be monitored prior to administration of each dose of this treatment (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients should be monitored during and after infusion (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Dose adjustments _Neutropenia_ If neutropenia develops during treatment it should be managed by dose delays. See Table 1 and Table 2 below for appropriate dosing recommendations for monotherapy and combination therapy, respectively. (see also section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 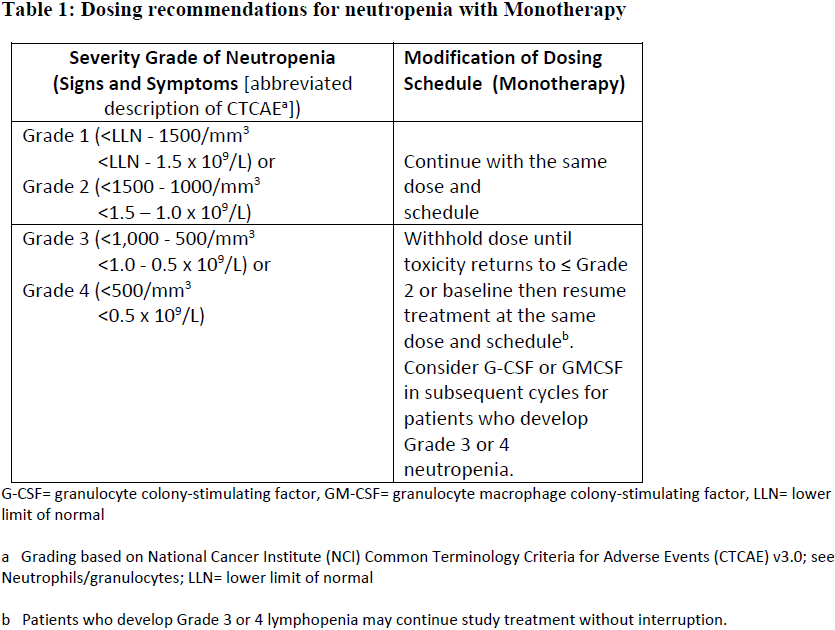 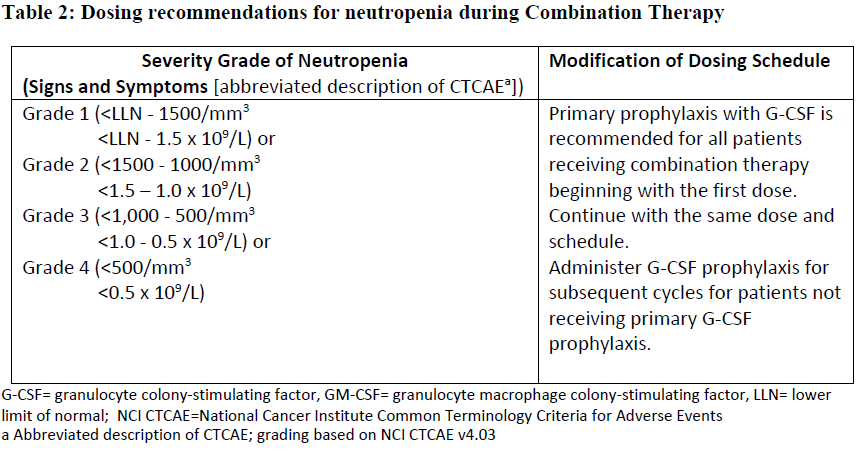 _Peripheral Neuropathy_ If peripheral sensory or motor neuropathy emerges or worsens during treatment see Table 3 and Table 4 below for appropriate dosing recommendations for monotherapy and combination therapy, respectively. (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 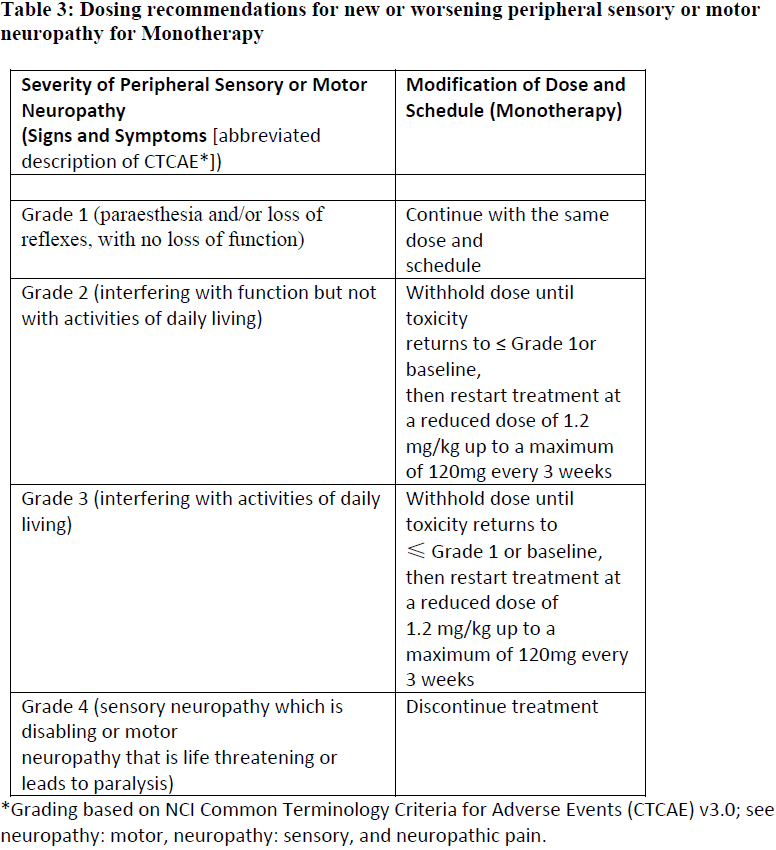 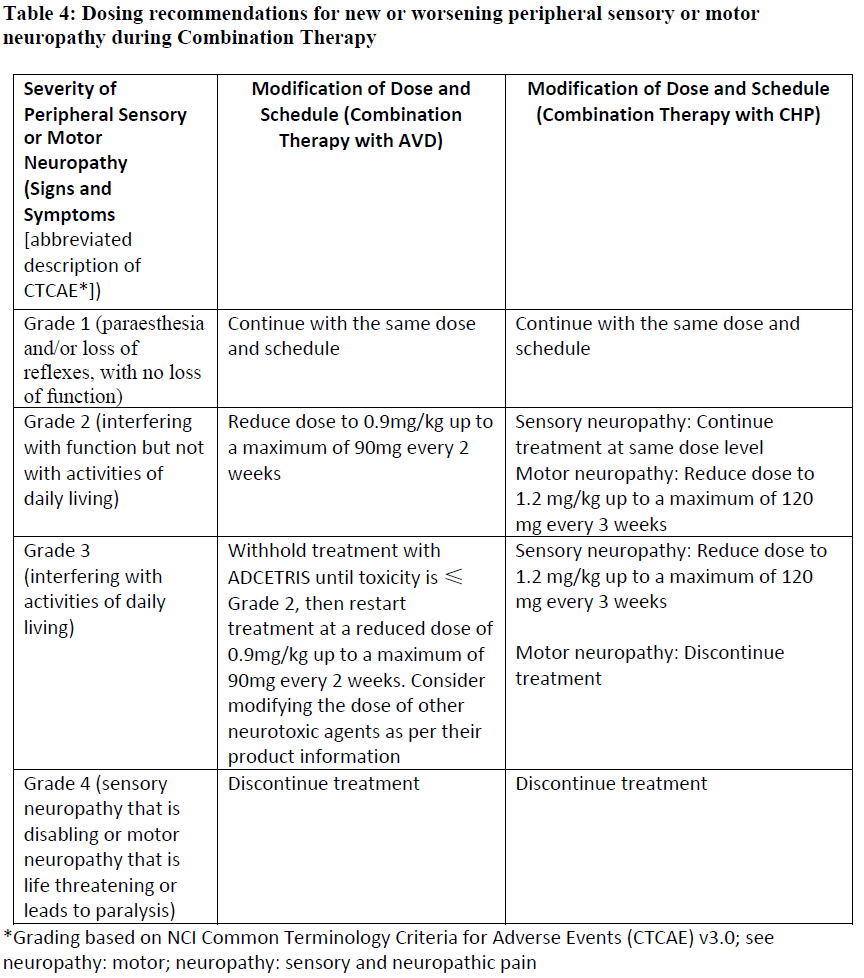 Special patient populations _Renal and hepatic impairment_ - Combination therapy Patients with renal impairment should be closely monitored for adverse events. There is no clinical trial experience using ADCETRIS in combination with chemotherapy in patients with renal impairment, where serum creatinine is ≥ 2.0 mg/dL and/or creatinine clearance or calculated creatinine clearance is ≤ 40 mL/minute. Use of ADCETRIS in combination with chemotherapy should be avoided in patients with severe renal impairment. Patients with hepatic impairment should be closely monitored for adverse events. The recommended starting dose in patients with mild hepatic impairment receiving ADCETRIS in combination with AVD is 0.9 mg/kg (up to a maximum of 90 mg) administered as an intravenous infusion over 30 minutes every 2 weeks. The recommended starting dose in patients with mild hepatic impairment receiving ADCETRIS in combination with CHP is 1.2 mg/kg administered as an intravenous infusion over 30 minutes every 3 weeks. There is no clinical trial experience using ADCETRIS in combination with chemotherapy in patients with hepatic impairment, where total bilirubin is > 1.5 times the upper limit of normal (ULN) (unless due to Gilbert syndrome), or aspartate aminotransferase (AST) or alanine aminotransferase (ALT) are > 3 times the ULN, or > 5 times the ULN if their elevation may be reasonably ascribed to the presence of HL in the liver. Use of ADCETRIS in combination with chemotherapy should be avoided in patients with moderate and severe hepatic impairment. - Monotherapy The recommended starting dose in patients with severe renal impairment is 1.2 mg/kg administered as an intravenous infusion over 30 minutes every 3 weeks. Patients with renal impairment should be closely monitored for adverse events (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The recommended starting dose in patients with hepatic impairment is 1.2 mg/kg administered as an intravenous infusion over 30 minutes every 3 weeks. Patients with hepatic impairment should be closely monitored for adverse events (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Elderly patients_ The dosing recommendations for patients aged 65 and older are the same as for adults. Currently available data are described in sections 4.8, 5.1 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. _Paediatric population_ The safety and efficacy of children less than 18 years have not yet been established. Currently available data are described in Pharmacokinetics (see section number 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). In nonclinical studies, thymus depletion has been observed (see section 5.3 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Method of administration The recommended dose of ADCETRIS is infused over 30 minutes. For instructions on reconstitution and dilution of the medicinal product before administration, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. ADCETRIS must not be administered as an intravenous push or bolus. ADCETRIS should be administered through a dedicated intravenous line and it must not be mixed with other medicinal products (see section 6.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
INTRAVENOUS DRIP
Medical Information
**_4.1 Therapeutic indications_** **4.1.1 Previously untreated Stage III or IV classical Hodgkin lymphoma (cHL), in combination with chemotherapy.** ADCETRIS is indicated for the frontline treatment of adult patients with previously untreated CD30+ advanced cHL in combination with doxorubicin, vinblastine and dacarbazine (AVD)(see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **4.1.2 Hodgkin lymphoma (HL) consolidation.** ADCETRIS is indicated for the treatment of adult patients with CD30+ HL at increased risk of relapse or progression following autologous stem cell transplant (ASCT) (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **4.1.3 Relapsed or refractory Hodgkin lymphoma (HL).** ADCETRIS is indicated for the treatment of adult patients with relapsed or refractory CD30+ Hodgkin lymphoma (HL): 1. following autologous stem cell transplant (ASCT) or 2. following at least two prior therapies when ASCT or multi-agent chemotherapy is not a treatment option. **4.1.4 Frontline systemic anaplastic large cell lymphoma (sALCL) or other CD30-expressing peripheral T-cell lymphomas (PTCL), in combination with chemotherapy.** ADCETRIS is indicated for the treatment of patients with CD30-expressing previously untreated peripheral T-cell lymphoma (PTCL) in combination with chemotherapy. **4.1.5 Relapsed or refractory systemic anaplastic large cell lymphoma (sALCL).** ADCETRIS is indicated for the treatment of adult patients with relapsed or refractory systemic anaplastic large cell lymphoma (sALCL). **4.1.6 CD30+ cutaneous T-cell lymphoma (CTCL).** ADCETRIS is indicated for the treatment of adult patients with CD30+ cutaneous T-cell lymphoma (CTCL) after at least 1 prior systemic therapy (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Combined use of bleomycin and ADCETRIS causes pulmonary toxicity.
L01XC12
xl 01 xc 12
Manufacturer Information
TAKEDA PHARMACEUTICALS (ASIA PACIFIC) PTE. LTD.
BSP PHARMACEUTICALS S.P.A
FAREVA PAU 2
Active Ingredients
Documents
Package Inserts
Adcetris powder for concentrate for solution for infusion 50mg per vial PI.pdf
Approved: February 22, 2023
