Basic Information
SAXENDA® SOLUTION FOR INJECTION IN PRE-FILLED PEN 6MG/ML
INJECTION, SOLUTION
Regulatory Information
SIN15338P
October 9, 2017
Prescription Only
Therapeutic
SUBCUTANEOUS
August 10, 2023
May 30, 2025
Company Information
NOVO NORDISK PHARMA (SINGAPORE) PTE LTD
NOVO NORDISK PHARMA (SINGAPORE) PTE LTD
Active Ingredients
Strength: 6.0 mg/ml
Detailed Information
Contraindications
**4.3 Contraindications** Hypersensitivity to liraglutide or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
Indication Information
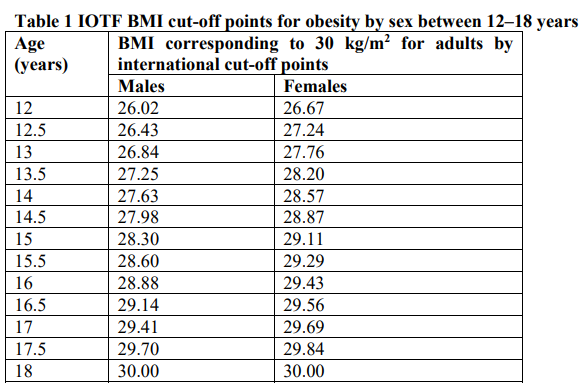
**4.1 Therapeutic indications** Adults Saxenda® is indicated as an adjunct to a reduced-calorie diet and increased physical activity for weight management in adult patients with an initial Body Mass Index (BMI) of - ≥30 kg/m2 (obesity), or - ≥27 kg/m2 to <30 kg/m2 (overweight) in the presence of at least one weight-related comorbidity such as dysglycaemia (prediabetes or type 2 diabetes mellitus), hypertension, dyslipidaemia or obstructive sleep apnoea. Treatment with Saxenda® should be discontinued after 12 weeks on the 3.0 mg/day dose if patients have not lost at least 5% of their initial body weight. Adolescents (≥12 years) Saxenda® can be used as an adjunct to a healthy nutrition and increased physical activity for weight management in adolescent patients from the age of 12 years and above with: - an inadequate response to reduced calorie diet and increased physical activity alone, and - obesity (BMI corresponding to ≥30 kg/m2 for adults by international cut-off points)\* and - body weight above 60 kg. Limitations of Use: The safety and effectiveness of Saxenda® in pediatric patients with type 2 diabetes have not been established. Treatment with Saxenda® should be discontinued and re-evaluated if patients have not lost at least 4% of their BMI or BMI z score after 12 weeks on the 3.0 mg/day or maximum tolerated dose. \*IOTF BMI cut-off points for obesity by sex between 12–18 years (see table 1):