Diphenoxylate Hydrochloride and Atropine Sulfate
Diphenoxylate Hydrochloride and Atropine Sulfate Tablets, for oral use C-V
83bcc2fb-6fb0-4f0e-8e66-592d327684ec
HUMAN PRESCRIPTION DRUG LABEL
Sep 12, 2023
Greenstone LLC
DUNS: 825560733
Mylan Pharmaceuticals Inc.
DUNS: 059295980
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Diphenoxylate Hydrochloride and Atropine Sulfate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Label
NDC 59762-1061-1
100 Tablets
GREENSTONE® BRAND
diphenoxylate
hydrochloride
and atropine
sulfate tablets,
USP
CV
2.5 mg/0.025 mg*
Rx only

DESCRIPTION SECTION
DESCRIPTION
Each diphenoxylate hydrochloride and atropine sulfate tablet contains:
2.5 mg of diphenoxylate hydrochloride USP (equivalent to 2.3 mg of diphenoxylate) and 0.025 mg of atropine sulfate USP (equivalent to 0.01 mg of atropine)
Diphenoxylate hydrochloride, an antidiarrheal, is ethyl 1-(3-cyano-3,3-diphenylpropyl)-4-phenylisonipecotate monohydrochloride and has the following structural formula:
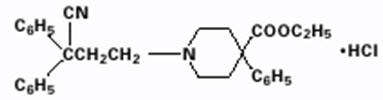
Atropine sulfate, an anticholinergic, is endo-(±)-α-(hydroxymethyl) benzeneacetic acid 8-methyl-8-azabicyclo[3.2.1] oct-3-yl ester sulfate (2:1) (salt) monohydrate and has the following structural formula:
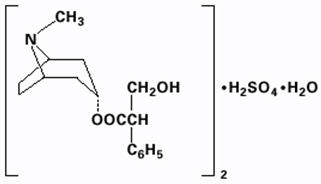
A subtherapeutic amount of atropine sulfate is present to discourage deliberate overdosage.
Inactive ingredients of diphenoxylate hydrochloride and atropine sulfate tablets include acacia, corn starch, magnesium stearate, sorbitol, sucrose, and talc.
SPL UNCLASSIFIED SECTION
This product's label may have been updated. For current full prescribing information, please visit www.greenstonellc.com.

LAB-0517-4.0
Revised March 2018
