Thiamine Hydrochloride
Thiamine Hydrochloride Injection, USP Rx only
2e957a35-83b3-47f5-ad09-216b22cc30be
HUMAN PRESCRIPTION DRUG LABEL
Nov 11, 2024
ONESOURCE SPECIALTY PHARMA LIMITED
DUNS: 867530307
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Thiamine Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
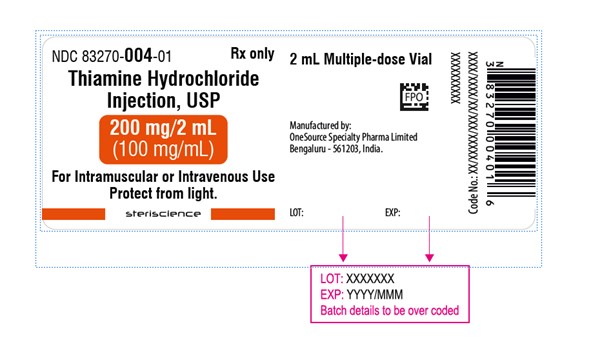
NDC 83270-004-01** Rx only**
**Thiamine Hydrochloride**
Injection, USP
200 mg per 2 mL
(100 mg per mL)
For Intramuscular or Intravenous use.
Protect from light.
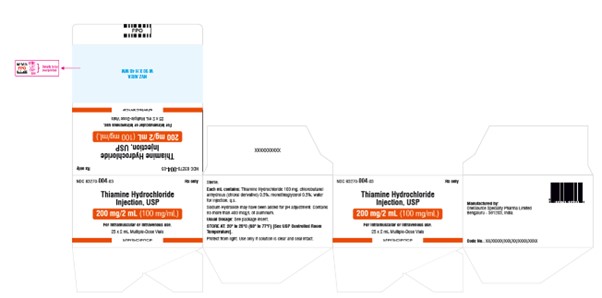
NDC 83270-004-03Rx only
Thiamine Hydrochloride
Injection, USP
200 mg per 2 mL (100 mg per mL)
For Intramuscular or Intravenous use.
25 x 2 mL Multiple-Dose Vials
DESCRIPTION SECTION
DESCRIPTION
Thiamine Hydrochloride Injection, USP is a sterile solution of thiamine hydrochloride in Water for Injection for intramuscular (IM) or slow intravenous (IV) administration.
Each mL contains: Thiamine hydrochloride 100 mg; chlorobutanol anhydrous (chloral derivative) 0.5%; monothioglycerol 0.5%; water for injection, q.s. Sodium hydroxide may have been added for pH adjustment (2.5 to 4.5).
Thiamine hydrochloride, or vitamin B 1, occurs as white crystals or crystalline powder that usually has a slight characteristic odor. Freely soluble in water; soluble in glycerin; slightly soluble in alcohol; insoluble in ether and benzene. Thiamine is rapidly destroyed in neutral or alkaline solutions but is stable in the dry state. It is reasonably stable to heat in acid solution.
The chemical name of thiamine hydrochloride is thiazolium,3-[(4-amino-2-methyl-5 pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylchloride, monohydrochloride and it has the following structural formula:

C12H17ClN4OS . HCl M.W. 337.27
