Clotrimazole and Betamethasone Dipropionate
These highlights do not include all the information needed to use CLOTRIMAZOLE AND BETAMETHASONE DIPROPIONATE CREAM safely and effectively. See full prescribing information for CLOTRIMAZOLE AND BETAMETHASONE DIPROPIONATE CREAM. CLOTRIMAZOLE and BETAMETHASONE DIPROPIONATE cream, for topical use Initial U.S. Approval: 1984
153fed41-6662-4ca9-802f-84b44dcb65e9
HUMAN PRESCRIPTION DRUG LABEL
Sep 9, 2025
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Clotrimazole and Betamethasone Dipropionate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Clotrimazole/Betamethasone Dipro Cream #45
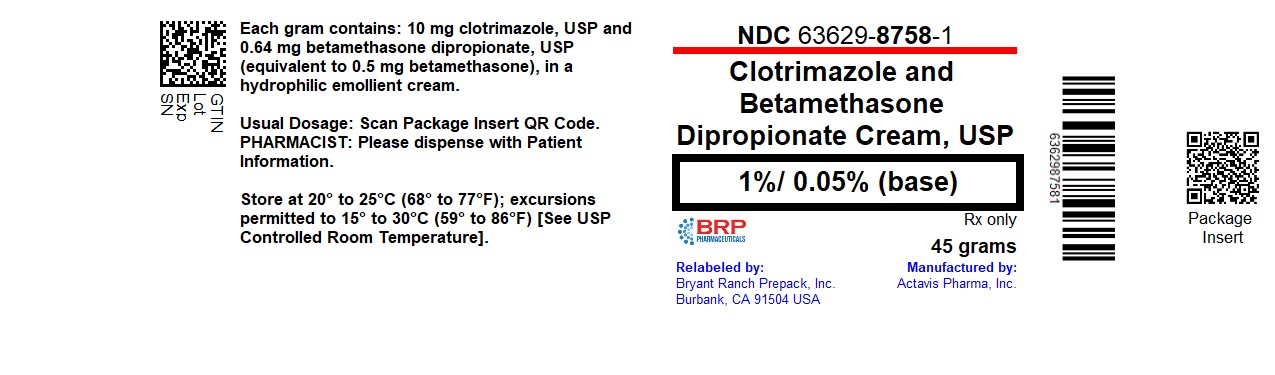
Label

DESCRIPTION SECTION
11 DESCRIPTION
Clotrimazole and Betamethasone Dipropionate Cream USP, 1%/0.05% (base), contains combinations of clotrimazole, USP, an azole antifungal, and betamethasone dipropionate, USP, a corticosteroid, for topical use.
Chemically, clotrimazole, USP is 1-(o-Chloro-α,α-diphenylbenzyl)imidazole, with the molecular formula C22H17ClN2, a molecular weight of 344.84, and the following structural formula:
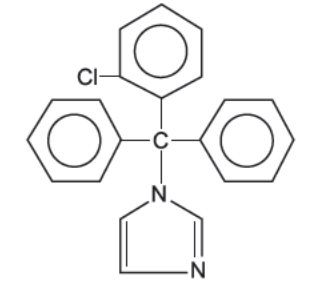
Clotrimazole, USP is an odorless, white crystalline powder, insoluble in water and soluble in ethanol.
Betamethasone dipropionate, USP has 9-Fluoro-11β,17,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate, with the molecular formula C28H37FO7, a molecular weight of 504.59, and the following structural formula:
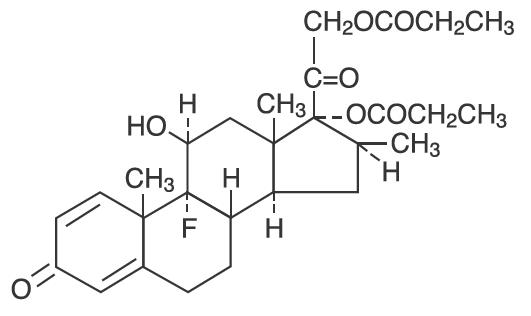
Betamethasone dipropionate, USP is a white to creamy-white, odorless crystalline powder, insoluble in water.
Each gram of Clotrimazole and Betamethasone Dipropionate Cream USP contains 10 mg clotrimazole, USP and 0.64 mg betamethasone dipropionate, USP (equivalent to 0.5 mg betamethasone), in a white to off-white hydrophilic cream. Inactive ingredients: Ceteareth-30, cetyl alcohol, mineral oil, propylene glycol, purified water, sodium phosphate monobasic monohydrate, stearyl alcohol and white petrolatum; benzyl alcohol as preservative.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Clotrimazole and Betamethasone Dipropionate Cream USP is available as follows:
45 gram tube in a carton (NDC: 63629-8758-1)
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.Burbank, CA 91504
