SELEGILINE HYDROCHLORIDE
Selegiline Hydrochloride Capsules, 5 mg
1924db3d-6a16-4496-cfd0-6903be146925
HUMAN PRESCRIPTION DRUG LABEL
Sep 22, 2025
Apotex Corp.
DUNS: 845263701
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
SELEGILINE HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (18)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL SECTION
Representative sample of the labeling (see theHOW SUPPLIED section for complete listing):
PRINCIPAL DISPLAY PANEL - 5 mg BOTTLE LABEL
**APOTEX CORP.**NDC 60505-0055-1
SELEGILINE HYDROCHLORIDE CAPSULES,
5 mg
Rx
60 Capsules

DESCRIPTION SECTION
DESCRIPTION
Selegiline hydrochloride is a levorotatory acetylenic derivative of phenethylamine. It is commonly referred to in the clinical and pharmacological literature as l- deprenyl.
The chemical name is: (R)-(-)-N,α-Dimethyl-N-2-propynylphenethylamine
hydrochloride. It is a white to near white crystalline powder, freely soluble
in water, chloroform, and methanol, and has a molecular weight of 223.75. The
molecular formula is C13H17N**.**HCI and the structural formula is as
follows: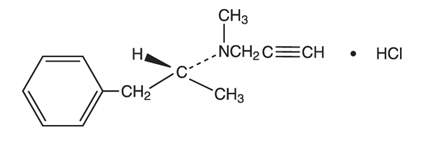
Each capsule, for oral administration contains 5 mg of selegiline hydrochloride. In addition, each capsule contains the following inactive ingredients: Anhydrous Lactose NF, Citric Acid Anhydrous USP, Microcrystalline Cellulose NF PH102, Stearic Acid NF and Talc USP. The capsule shell contains Gelatin NF, FD & C Blue #1 and Titanium Dioxide. The capsule logo ink Black SW-9008/SW-9009 contains the following inactive ingredients: Ammonium Hydroxide; Black Iron Oxide, Bacteria Controlled EEC No. 172; n-Butyl NF; Ethyl Alcohol, Anhydrous, 200 Proof; Isopropyl Alcohol USP; Potassium Hydroxide NF; Propylene Glycol USP; Purified Water USP and Shellac NF.
