One Natal Rx
OneNatal RX Rx Prenatal Vitamin TabletIron with Prescription Folic AcidPrenatal & Postnatal
a4f80fca-e532-42a9-a6ae-125925cfb3f1
DIETARY SUPPLEMENT
Aug 20, 2025
AARNA USA INC
DUNS: 118515992
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Vitamin A, Ascorbic Acid, Cholecalciferol, DL-Alpha Tocopheryl Acetate, Thiamine Mononitrate, Vitamin B2, Niacinamide, Pyridoxine HCl, Folic Acid, Cyanocobalamin, Calcium Carbonate, Ferrous Fumarate, Zinc Oxide, Cupric Oxide, Inositol, Citrus Bioflavonoids Fruit Complex, Boron Citrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (27)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
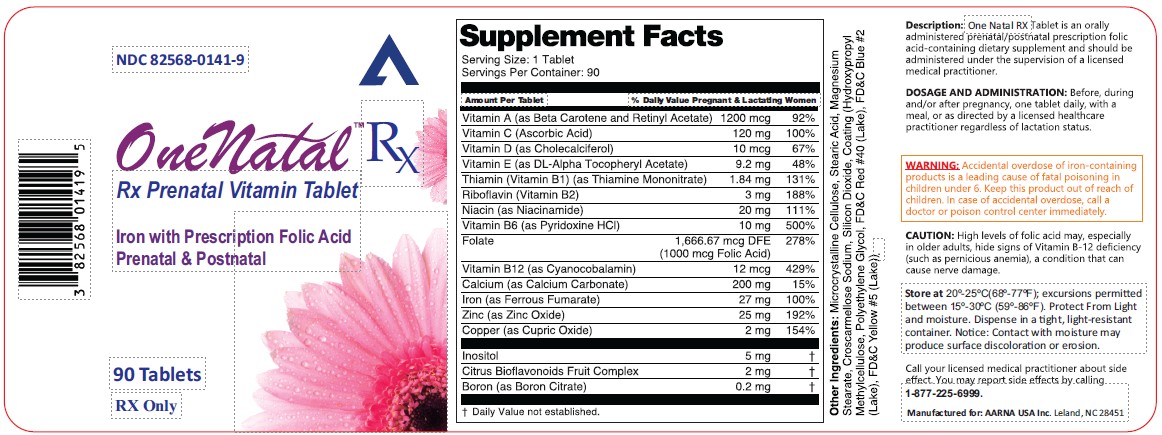
STATEMENT OF IDENTITY SECTION
HEALTH CLAIM SECTION
|
Supplement Facts | ||
|
Serving Size: 1 Tablet | ||
|
Servings Per Container: 90 | ||
|
Amount Per Tablet |
% Daily Value Pregnant & Lactating Women | |
|
Vitamin A (as Beta Carotene and Retinyl Acetate) |
1200 mcg |
92% |
|
Vitamin C (Ascorbic Acid) |
120 mg |
100% |
|
Vitamin D (as Cholecalciferol) |
10 mcg |
67% |
|
Vitamin E (as DL-Alpha Tocopheryl Acetate) |
9.2 mg |
48% |
|
Thiamin (Vitamin B1) (as Thiamine Mononitrate) |
1.84 mg |
131% |
|
Riboflavin (Vitamin B2) |
3 mg |
188% |
|
Niacin (as Niacinamide) |
20 mg |
111% |
|
Vitamin B6 (as Pyridoxine HCl) |
10 mg |
500% |
|
Folate |
1,666.67 mcg DFE |
278% |
|
Vitamin B12 (as Cyanocobalamin) |
12 mcg |
429% |
|
Calcium (as Calcium Carbonate) |
200 mg |
15% |
|
Iron (as Ferrous Fumarate) |
27 mg |
100% |
|
Zinc (as Zinc Oxide) |
25 mg |
192% |
|
Copper (as Cupric Oxide) |
2 mg |
154% |
|
Inositol |
5 mg |
† |
|
Citrus Bioflavonoids Fruit Complex |
2 mg |
† |
|
Boron (as Boron Citrate) |
0.2 mg |
† |
|
† Daily Value not established. |
Other Ingredients: Microcrystalline Cellulose, Stearic Acid, Magnesium Stearate, Croscarmellose Sodium, Silicon Dioxide, Coating (Hydroxypropyl Methylcellulose, Polyethylene Glycol, FD&C Red #40 (Lake), FD&C Blue #2 (Lake), FD&C Yellow #5(Lake)).
Description:
One Natal RX Tablet is an orally administered prenatal/postnatal prescription folic acid-containing dietary supplement and should be administered under the supervision of a licensed medical practitioner.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION:
Before, during and/or after pregnancy, one tablet daily, with a meal, or as directed by a licensed healthcare practitioner regardless of lactation status.
WARNINGS SECTION
WARNING:
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
SAFE HANDLING WARNING SECTION
CAUTION:
High levels of folic acid may, especially in older adults, hide signs of Vitamin B-12 deficiency (such as pernicious anemia), a condition that can cause nerve damage.
Store at 15°-30° C (59°-86° F). Protect from light and moisture. Dispense in a tight, light-resistant container. Notice: Contact with moisture may produce surface discoloration or erosion.
Call your licensed medical practitioner about side effect. You may report side effects by calling1-877-225-6999.
Manufactured for:
AARNA USA Inc.
Leland, NC 28451
