Tagrid Skin Repair Cream
Initial Drug Listing-Tagrid Skin Repair Ointment Allantoin 1%
34a8bf10-1c18-1c14-e063-6394a90a0a6f
HUMAN OTC DRUG LABEL
May 8, 2025
TAGRID LLC
DUNS: 118778969
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Allantoin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
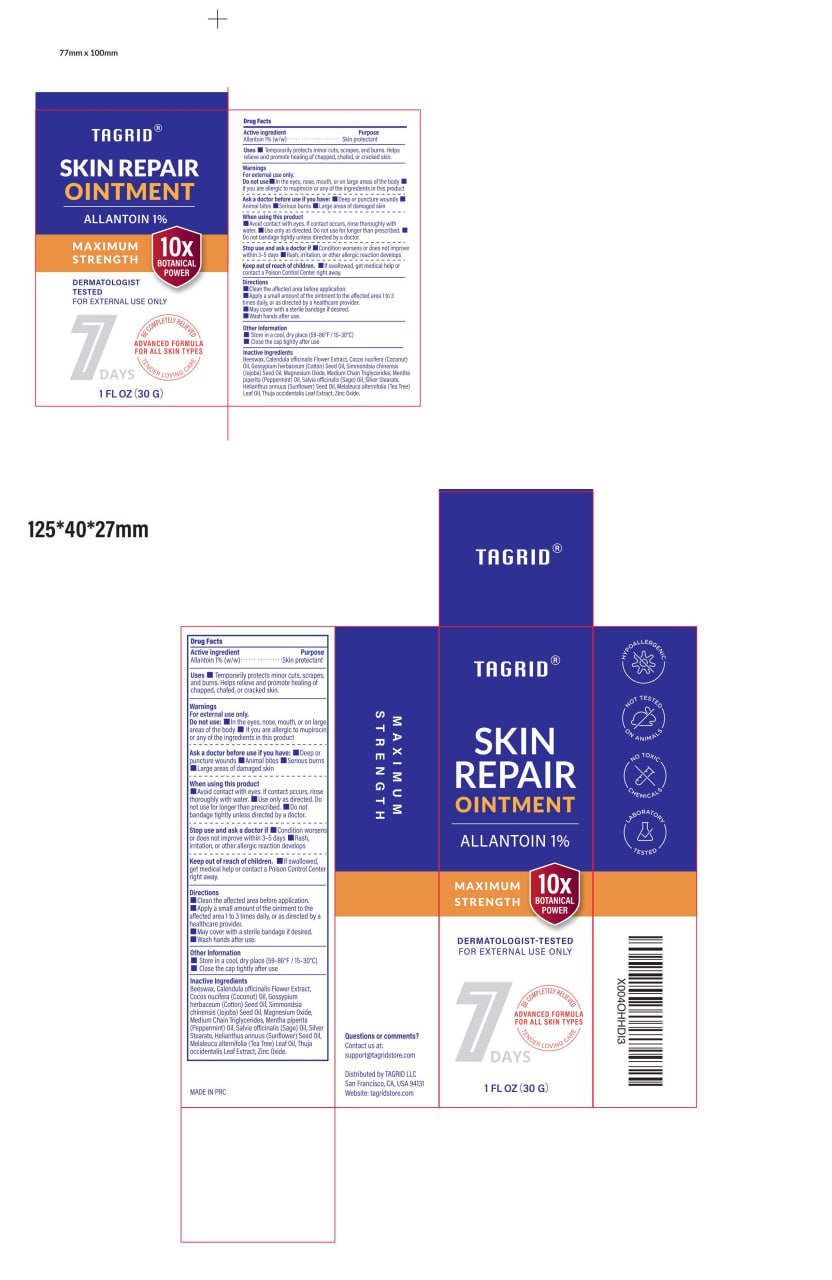
INDICATIONS & USAGE SECTION
Temporarily protects minor cuts, scrapes, and burns. Helps relieve and promote healing of chapped, chafed, or cracked skin.
OTC - ACTIVE INGREDIENT SECTION
Allantoin 1% (w/w/)
OTC - PURPOSE SECTION
Skin protectant
WARNINGS SECTION
For external use only
Do not use: In the eyes, nose, mouth, or on large
areas of the body
If you are allergic to mupirocin or any of the ingredients in this product
Ask a doctor before use if you have:
Deep or puncture wounds
Animal bites
Serious burns
Large areas of damaged skin
■ Avoid contact with eyes. If contact occurs, rinse thoroughly with water.
■ Use only as directed. Do not use for longer than prescribed.
■ Do not bandage tightly unless directed by a doctor.
Stop use and ask a doctor if
Condition worsens or does not improve within 3-5 days
Rash,irritation, or other allergic reaction develops
If swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
Clean the affected area before application.
Apply a small amount of the ointment to the affected area 1 to 3 times daily,
or as directed by a
healthcare provider.
May cover with a sterile bandage if desired.
Wash hands after use.
OTHER SAFETY INFORMATION
Store in a cool, dry place (59-86°F/15-30°C)
Close the cap tightly after use
OTC - QUESTIONS SECTION
Questions or comments?
Contact us at:
support@tagridstore.com
Distributed by TAGRID LLC
San Francisco, CA, USA 94131
Website: tagridstore.com
INACTIVE INGREDIENT SECTION
Beeswax,
Calendula officinalis Flower Extract,
Cocos nucifera (Coconut) Oil,
Gossypium herbaceum (Cotton) Seed Oil,
Simmondsia chinensis (Jojoba) Seed Oil,
Magnesium Oxide,
Medium Chain Triglycerides,
Mentha piperita
(Peppermint) Oil,
Salvia officinalis (Sage) Oil,
Silver Stearate,
Helianthus annuus (Sunflower) Seed Oil,
Melaleuca alternifolia (Tea Tree) Leaf Oil,
Thuja occidentalis Leaf Extract,
Zinc Oxide.
