Clobetasol Propionate
Clobetasol Propionate Cream, USP, 0.05%
6d14d269-bae6-493a-81ed-83f6a3aa0f4f
HUMAN PRESCRIPTION DRUG LABEL
Sep 3, 2025
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Clobetasol Propionate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
Drug Labeling Information
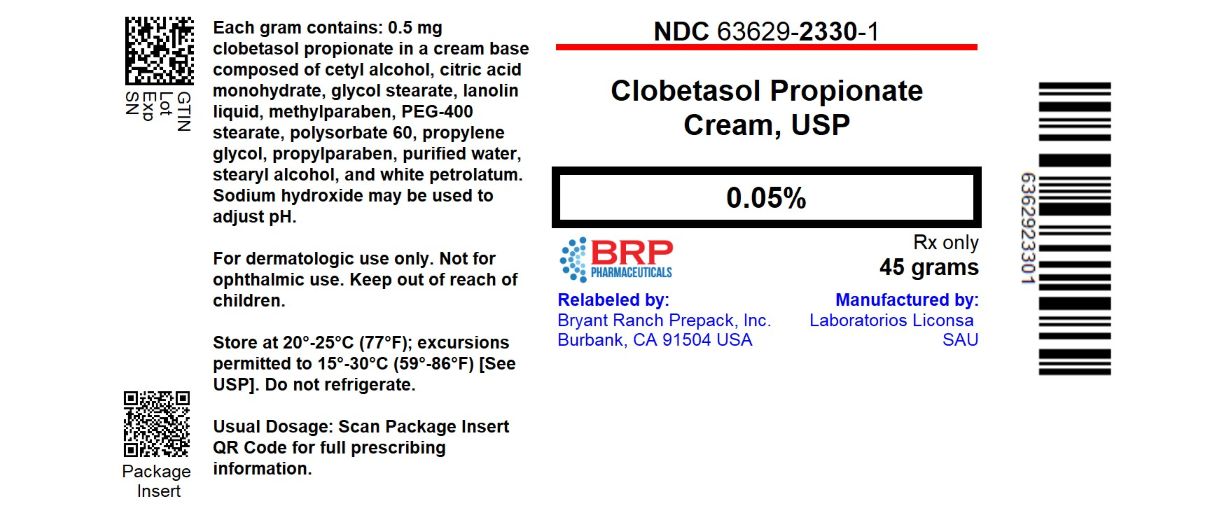
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Clobetasol Propionate 0.05% Cream
45 grams
HOW SUPPLIED SECTION
HOW SUPPLIED
Clobetasol Propionate Cream, USP, 0.05% is supplied in 45-g tubes (NDC 63629-2330-1).
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Clobetasol propionate cream should not be refrigerated.
Rx only
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
DESCRIPTION SECTION
DESCRIPTION
Clobetasol Propionate Cream USP, 0.05% contains the active compound clobetasol propionate, a synthetic corticosteroid, for topical dermatologic use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree of mineralocorticoid activity.
Chemically, clobetasol propionate is (11ß,16ß)-21-chloro-9-fluoro-11-hydroxy-16-methyl-17-(1-oxopropoxy)-pregna-1,4- diene-3,20-dione, and it has the following structural formula:
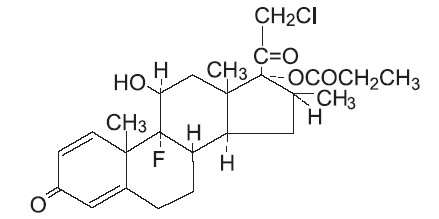
Clobetasol propionate has the molecular formula C25H32CIFO5 and a molecular weight of 467. It is a white to cream-colored crystalline powder insoluble in water.
Clobetasol propionate cream contains clobetasol propionate 0.5 mg/g in a cream base composed of cetyl alcohol, citric acid monohydrate, glycol stearate, lanolin liquid, methylparaben, PEG-400 stearate, polysorbate 60, propylene glycol, propylparaben, purified water, stearyl alcohol, and white petrolatum. Sodium hydroxide may be used to adjust pH.
