Desmopressin Acetate
Desmporessin Acetate Tablets 0.1 mg, 0.2 mg
72aeef35-c102-b628-0219-9822651887eb
HUMAN PRESCRIPTION DRUG LABEL
Sep 17, 2025
Apotex Corp.
DUNS: 845263701
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
desmopressin acetate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
desmopressin acetate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 0.2 mg BOTTLE LABEL
APOTEX CORP. NDC 60505-0258-3
Desmopressin Acetate Tablets
0.2 mg
Rx
30 bottle count

DESCRIPTION SECTION
DESCRIPTION
Desmopressin Acetate Tablets are a synthetic analogue of the natural pituitary hormone 8-arginine vasopressin (ADH), an antidiuretic hormone affecting renal water conservation. It is chemically defined as follows:
Mol. Wt. 1129.27
Empirical Formula: C46H64N14O12S2 • C2H4O2
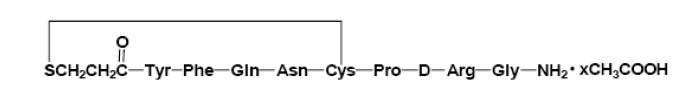
where 1<x<1.5
1-(3-mercaptopropionic acid)-8-D-arginine vasopressin monoacetate (salt).
Desmopressin acetate tablets, for oral administration, contain either 0.1 or 0.2 mg desmopressin acetate. In addition, each tablet contains the following inactive ingredients: anhydrous lactose, corn starch, and magnesium stearate.
SPL UNCLASSIFIED SECTION
APOTEX INC.
DESMOPRESSIN ACETATE TABLETS
0.1 mg and 0.2 mg
Manufactured by: Manufactured for:
Apotex Inc. Apotex Corp.
Toronto, Ontario Weston, Florida
Canada M9L 1T9 33326
Revised: October 2008
Rev. 4
