Mucus Relief D
Meijer 44-547
383281d7-0d25-4423-ace2-d2985f2c37d0
HUMAN OTC DRUG LABEL
Aug 26, 2025
Meijer Distribution Inc
DUNS: 006959555
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Guaifenesin, Pseudoephedrine HCl
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel
NDC 41250-547-72
meijer®
mucus
****relief D
****Guaifenesin | Pseudoephedrine HCl
Expectorant | Nasal Decongestant
27 Tablets | actual size
Clears Nasal/
Sinus Congestion
Thins & Loosens Mucus
Immediate Release
TAMPER EVIDENT: DO NOT USE IF
** PACKAGE IS OPENED OR IF BLISTER**
** UNIT IS TORN, BROKEN OR SHOWS**
** ANY SIGNS OF TAMPERING**
50844 REV0419A54772
DIST. BY MEIJER
** DISTRIBUTION, INC.**
** GRAND RAPIDS, MI 49544**
****www.meijer.com
OUR QUALITY
** GUARANTEE**
The Meijer
** Family******
********WWW.MEIJER.COM/SATISFACTION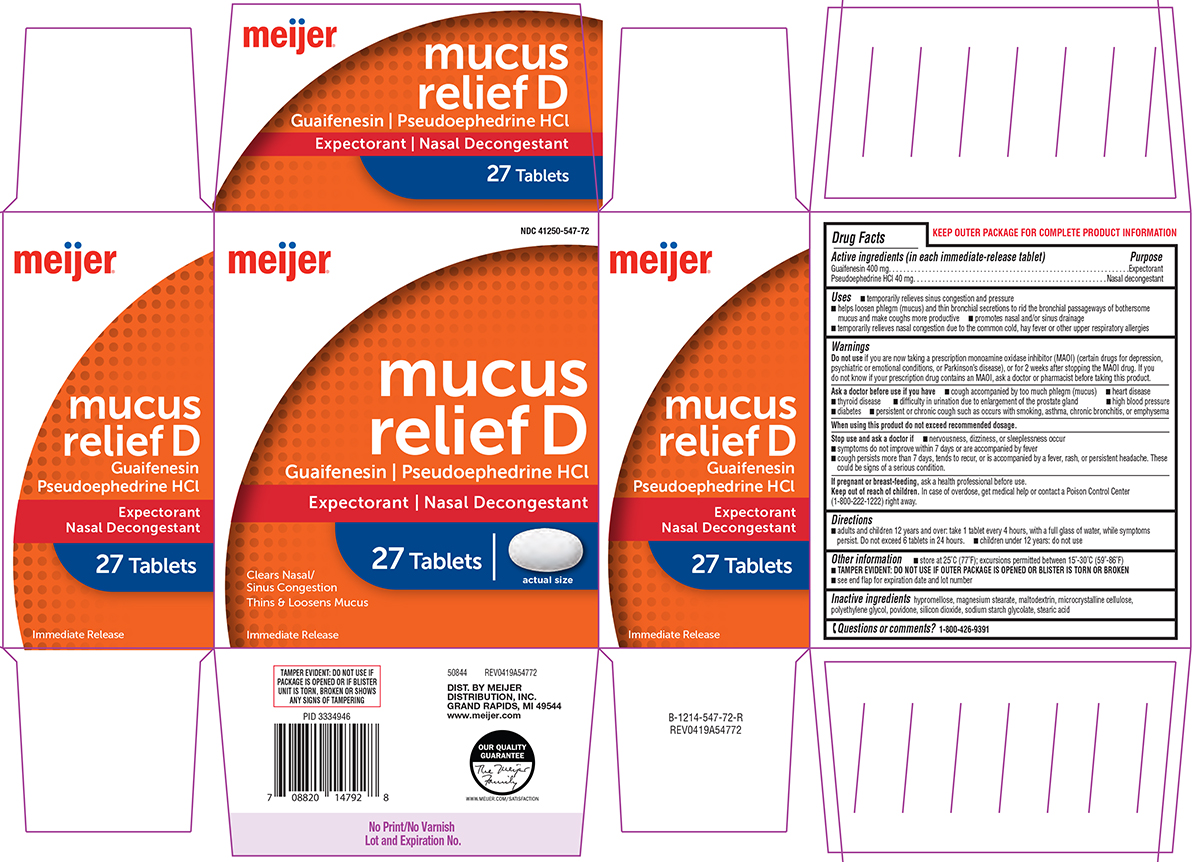
Meijer 44-547
INDICATIONS & USAGE SECTION
Uses
- temporarily relieves sinus congestion and pressure
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- promotes nasal and/or sinus drainage
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
OTC - ACTIVE INGREDIENT SECTION
Active ingredients****(in each immediate-release tablet)****
Guaifenesin 400 mg
Pseudoephedrine HCl 40 mg
OTC - PURPOSE SECTION
Purpose
Expectorant
Nasal decongestant
WARNINGS SECTION
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- cough accompanied by too much phlegm (mucus)
- heart disease
- thyroid disease
- difficulty in urination due to enlargement of the prostate gland
- high blood pressure
- diabetes
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
When using this product
do not exceed recommended dosage.
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- symptoms do not improve within 7 days or are accompanied by fever
- cough persists more than 7 days, tends to recur, or is accompanied by a fever, rash, or persistent headache. These could be signs of a serious condition.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
DOSAGE & ADMINISTRATION SECTION
Directions
- adults and children 12 years and over: take 1 tablet every 4 hours, with a full glass of water, while symptoms persist. Do not exceed 6 tablets in 24 hours.
- children under 12 years: do not use
STORAGE AND HANDLING SECTION
Other information
- store at 25ºC (77ºF); excursions permitted between 15°-30°C (59°-86°F) *TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- see end flap for expiration date and lot number
INACTIVE INGREDIENT SECTION
Inactive ingredients
hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, povidone, silicon dioxide, sodium starch glycolate, stearic acid
OTC - QUESTIONS SECTION
Questions or comments?
1-800-426-9391
