Sensitive Extra Whitening
5820075 Publix Sensitive Extra Whitening (8015201)
16eddce3-03e4-4c0e-e063-6294a90ae94d
HUMAN OTC DRUG LABEL
Aug 12, 2025
Publix Super Market
DUNS: 006922009
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Sodium Fluoride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
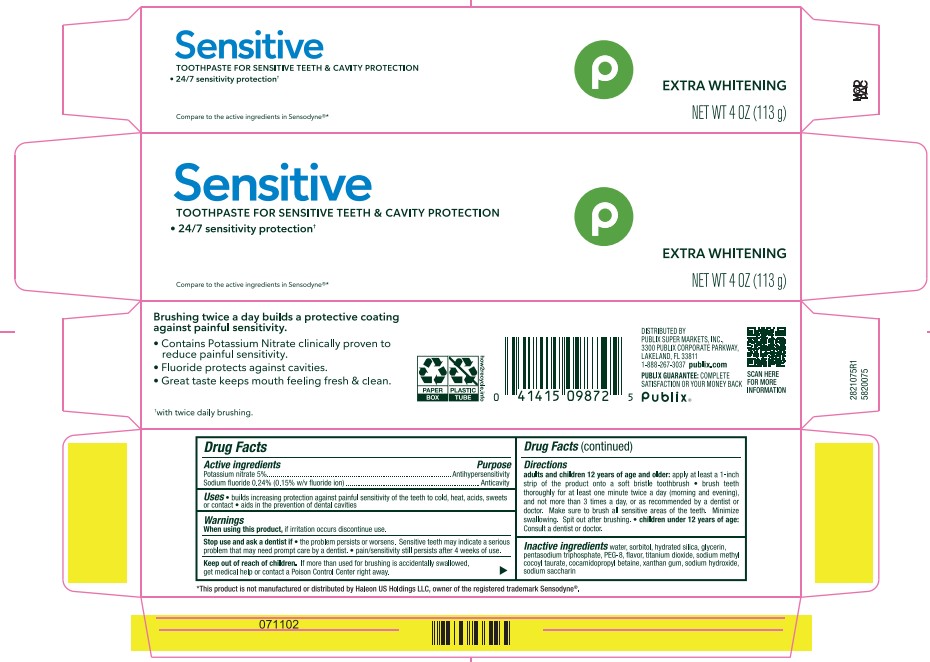
INDICATIONS & USAGE SECTION
builds increasing protection against painful sensitivity of the teeth to cold, heat, acids, sweets or contact * acids in the prevention of dental cavities
OTC - ACTIVE INGREDIENT SECTION
Potassium nitrate 5%
Sodium fluoride 0.24% (0.15% w/v fluoride ion)
OTC - PURPOSE SECTION
Antihypersensitivity
Anticavity
WARNINGS SECTION
When using this product, if irritation occurs discontinue.
Stop use and ask a dentist if * the problem persists or worsen. Sensitive teeth may indicate a serious problem that may need prompt care by a dentist. * pain/sensitivity still persists after 4 weeks of use.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Center right away.
DOSAGE & ADMINISTRATION SECTION
adults and children 12 years pf age and older: apply at least a 1-inch strip of the product onto a soft bristle toothbrush * brush teeth thoroughly for at least one minute twice a day (morning and evening), and not more than 3 times a day, or as recommended by a dentist or doctor. Make sure to brush all sensitive areas of the teeth. Minimize swallowing. Spit out after brushing. * children under 12 years of age: Consult a dentist or doctor.
INACTIVE INGREDIENT SECTION
water, sorbitol, hydrated silica, glycerin, pentasodium triphosphate, PEG-8, flavor, titanium dioixde, sodium methyl cocoyl taurate, cocamidopropyl betaine, xanthan gum, sodium hydroxide, sodium saccharin
