Liquid Glycerin Laxatives
Liquid Glycerin Laxatives
2dd97e1c-3f0b-6728-e063-6294a90abfa2
HUMAN OTC DRUG LABEL
Feb 19, 2025
Shanghai Yijing Medical Technology Co., Ltd.
DUNS: 567720668
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
GLYCERIN
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
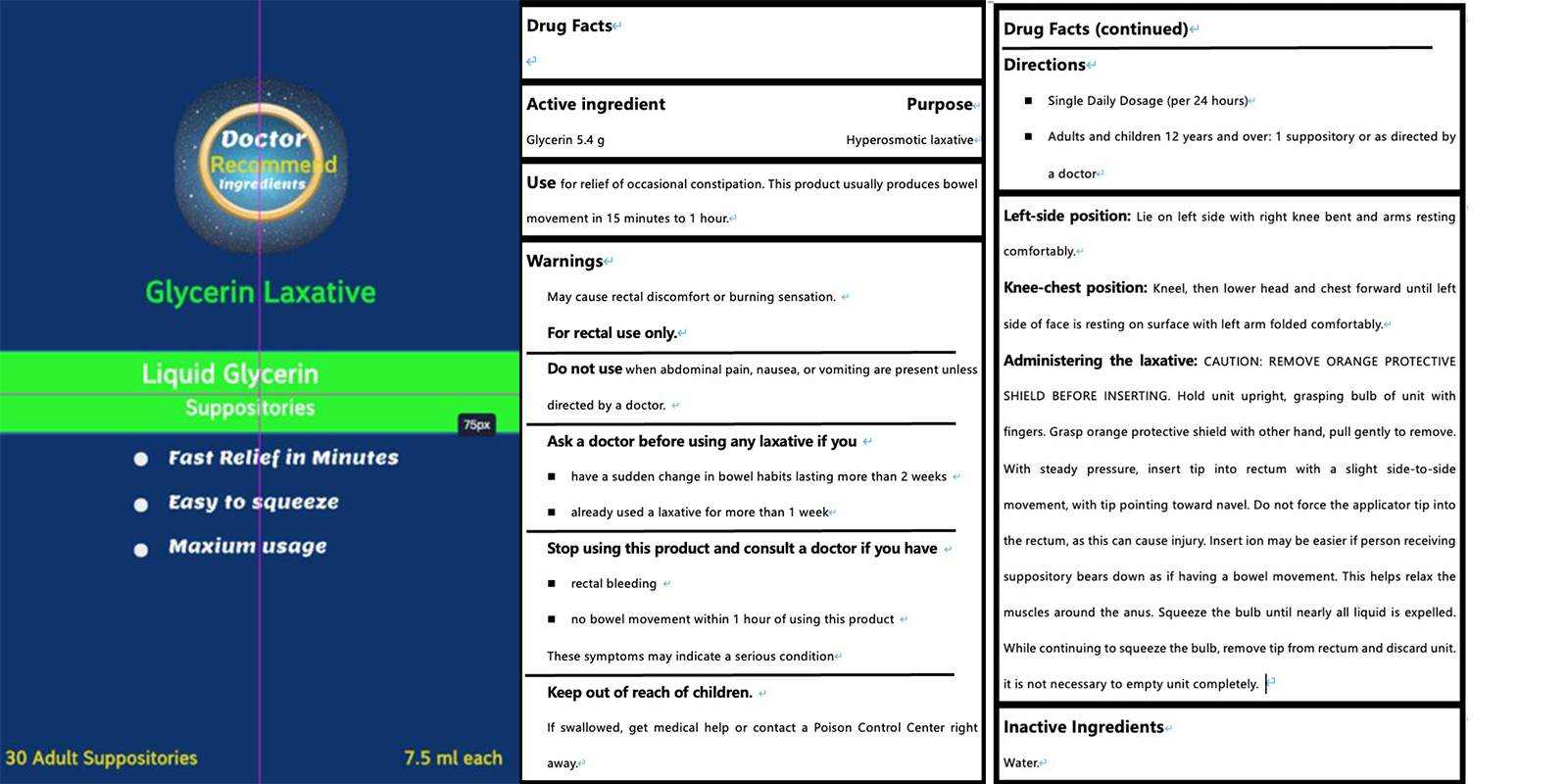
INDICATIONS & USAGE SECTION
USe for relief of occasional constipation. This product usually produces bowel movement in 15 minutes to 1 hour.
OTC - DO NOT USE SECTION
Do not use when abdominal pain, nausea, or vomiting are present unless directed by a doctor.
OTC - WHEN USING SECTION
Ask a doctor before using any laxative if you
have a sudden change in bowel habits lasting more than 2 weeks
already used a laxative for more than 1 week
OTC - STOP USE SECTION
Stop using this product and consult a doctor if you have
rectal bleeding
no bowel movement within 1 hour of using this product
These symptoms may indicate a serious condition
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
If swallowed, get medical help or contact a Poison Control Center right away.
SPL UNCLASSIFIED SECTION
Liquid Glycerin Laxatives
OTC - ACTIVE INGREDIENT SECTION
GLYCERIN 5.3g
OTC - PURPOSE SECTION
Hyperosmotic laxative
WARNINGS SECTION
May cause rectal discomfort or burning sensation.
For rectal use only
DOSAGE & ADMINISTRATION SECTION
Single Daily Dosage (per 24 hours)
Adults and children 12 years and over: 1 suppository or as directed by a
doctore
Left-side position: Lie on left side with right knee bent and arms resting
comfortably.
Knee-chest position: Kneel, then lower head and chest forward until leftside
of face is resting on surface with left arm folded comfortably. Administering
the laxative: CAUTION: REMOVE ORANGE PROTECTIVESHIELD BEFORE INSERTING. Hold
unit upright, grasping bulb of unit withfingers. Grasp orange protective
shield with other hand, pull gently to remove.With steady pressure, insert tip
into rectum with a slight side-to-sidemovement, with tip pointing toward
navel. Do not force the applicator tip intothe rectum, as this can cause
injury. Insert ion may be easier if person receivingsuppository bears down as
if having a bowel movement. This helps relax themuscles around the anus.
Squeeze the bulb until nearly all liquid is expelled.While continuing to
squeeze the bulb, remove tip from rectum and discard unit.
it is not necessary to empty unit completely.
INACTIVE INGREDIENT SECTION
WATER
