Tranylcypromine Sulfate
These highlights do not include all the information needed to use TRANYLCYPROMINE SULFATE TABLETS safely and effectively. See full prescribing information for TRANYLCYPROMINE SULFATE TABLETS. Tranylcypromine sulfate tablets, for oral use Initial U.S. Approval: 1961
6a0b609b-0625-4c26-91a1-59ee3ece3ddf
HUMAN PRESCRIPTION DRUG LABEL
Aug 27, 2021
Actavis Pharma, Inc.
DUNS: 119723554
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
tranylcypromine sulfate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
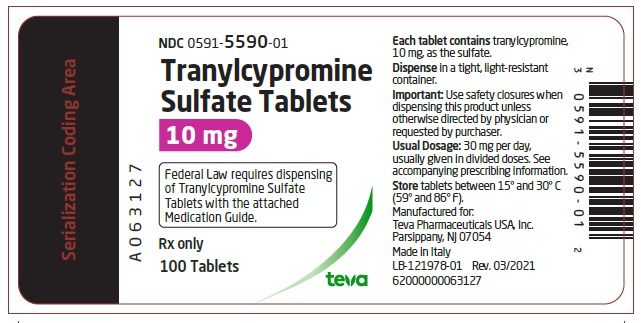
Bottle Label
NDC 0591-5590-01
Tranylcypromine Sulfate Tablets
10 mg
Federal Law requires dispensing of Tranylcypromine Sulfate Tablets with the attached Medication Guide.
Rx only
100 Tablets
teva
BOXED WARNING SECTION
WARNING:SUICIDAL THOUGHTS AND BEHAVIORS and HYPERTENSIVE CRISIS WITH
SIGNIFICANT TYRAMINE USE
INDICATIONS & USAGE SECTION
1 INDICATIONS & USAGE
Tranylcypromine sulfate tablets are indicated for the treatment of major depressive disorder (MDD) in adult patients who have not responded adequately to other antidepressants. Tranylcypromine sulfate tablets are not indicated for the initial treatment of MDD due to the potential for serious adverse reactions and drug interactions, and the need for dietary restrictions[see Contraindications (4),Warnings and Precautions (5), and Drug Interactions (7)].
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
4.1 Combination with Certain Drugs
Concomitant use of tranylcypromine sulfate tablets or use in rapid succession with the products in Table 1 is contraindicated. Such use may cause severe or life-threatening reactions such as hypertensive crises or serotonin syndrome [see Drug Interactions (7.1)].Medication-free periods between administration of tranylcypromine sulfate tablets and contraindicated agents are recommended [ see Dosage and Administration (2.2)and Drug Interactions (7.1) ].
Table 1: Products Contraindicated with the Use of Tranylcypromine Sulfate Tablets
|
Drug Classes | ||
|
Non-selective H1 receptor antagonists | ||
|
Antidepressants including but not limited to:
| ||
|
Amphetamines and methylphenidates and derivatives | ||
|
Sympathomimetic products (e.g., cold, hay fever or weight reducing products that contain vasoconstrictors such as pseudoephedrine, phenylephrine, and ephedrine; or dietary supplements that contain sympathomimetics) | ||
|
Triptans | ||
|
Individual Drugs (not included in the above classes) | ||
|
buspirone |
levodopa |
s-adenosyl-L-methionine (SAM-e) |
|
carbamazepine |
meperidine |
tapentadol |
|
cyclobenzaprine |
methyldopa |
tetrabenazine |
|
dextromethorphan |
milnacipran |
tryptophan |
|
dopamine |
rasagiline | |
|
hydroxytryptophan |
reserpine |
4.2 Pheochromocytoma and Catecholamine-Releasing Paragangliomas
Tranylcypromine sulfate tablets are contraindicated in the presence of pheochromocytoma or other catecholamine-releasing paragangliomas because such tumors secrete pressor substances and can lead to hypertensive crisis [see Warnings and Precautions (5.3)].
- Concomitant use or use in rapid succession with other MAOIs; selective serotonin reuptake inhibitors; serotonin and norepinephrine reuptake inhibitors; tricyclic antidepressants; sympathomimetic drugs; and numerous other drugs. See Full Prescribing Information for the full list of contraindicated products (4.1, 7.1)
- Pheochromocytoma, other catecholamine-releasing paraganglioma (4.2)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behaviors in Adolescents and Young Adults
In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that included approximately 77,000 adult patients and 4,500 pediatric patients, the incidence of suicidal thoughts and behaviors in antidepressant-treated patients age 24 years and younger was greater than in placebo-treated patients. There was considerable variation in risk of suicidal thoughts and behaviors among drugs, but there was an increased risk identified in young patients for most drugs studied. There were differences in absolute risk of suicidal thoughts and behaviors across the different indications, with the highest incidence in patients with MDD. The drug-placebo differences in the number of cases of suicidal thoughts and behaviors per 1000 patients treated are provided in Table 2.
Table 2: Risk Differences of the Number of Patients of Suicidal Thoughts and Behavior in the Pooled Placebo-Controlled Trials of Antidepressants in Pediatric and Adult Patients
|
Age Range |
Drug-Placebo Difference in Number of Patients of Suicidal Thoughts or Behaviors per 1000 Patients Treated |
|
Increases Compared to Placebo | |
|
<18 years old |
14 additional patients |
|
18-24 years old |
5 additional patients |
|
Decreases Compared to Placebo | |
|
25-64 years old |
1 fewer patient |
|
≥65 years old |
6 fewer patients |
It is unknown whether the risk of suicidal thoughts and behaviors in children, adolescents, and young adults extends to longer-term use, i.e., beyond four months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with MDD that antidepressants delay the recurrence of depression and that depression itself is a risk factor for suicidal thoughts and behaviors.
Monitor all antidepressant-treated patients for any indication for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy, and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Consider changing the therapeutic regimen, including possibly discontinuing tranylcypromine sulfate tablets , in patients whose depression is persistently worse, or who are experiencing emergent suicidal thoughts or behaviors.
5.2 Hypertensive Crisis and Hypertension
Hypertensive Crisis
MAOIs, including tranylcypromine sulfate tablets, have been associated with
hypertensive crises caused by the ingestion of foods or beverages with a high
concentration of tyramine. In addition, hypertensive reactions and crises may
occur with concomitant use of other drugs [see Drug Interactions (7.1)].
Patients with hyperthyroidism may be at greater risk of hypertensive crisis.
Signs, Symptoms, and Complications of Hypertensive Crisis: In some patients a hypertensive crisis constitutes a hypertensive emergency, which requires immediate attention to prevent serious complications or fatal outcome. These emergencies are characterized by severe hypertension (e.g., with a blood pressure of more than 180/120 mm Hg) and evidence of organ dysfunction. Symptoms may include occipital headache (which may radiate frontally), palpitations, neck stiffness or soreness, nausea or vomiting, sweating (sometimes with fever or cold, clammy skin), dilated pupils, photophobia, shortness of breath, or confusion. Either tachycardia or bradycardia may be present and may be associated with constricting chest pain. Seizures may also occur. Intracranial bleeding, sometimes fatal, has been reported in association with the increase in blood pressure.
Strategies to Reduce the Risk of Hypertensive Crisis: Instruct patients to avoid foods and beverages with high tyramine content while being treated with tranylcypromine sulfate tablets and for 2 weeks after stopping tranylcypromine sulfate tablets [see Drug Interactions (7.2)].Careful evaluation of the benefits and risks of tranylcypromine sulfate tablets therapy is necessary in patients with:
- Hypertension or confirmed or suspected cerebrovascular or cardiovascular disorders that constitute an increased risk for complications from severe hypertension, and
- A history of headaches that can mask the occurrence of headaches as prodromal of a hypertensive crisis.
In all patients taking tranylcypromine sulfate tablets, monitor blood pressure closely to detect evidence of increased blood pressure. Full reliance should not be placed on blood pressure readings. The patient should also be observed for other signs and symptoms of hypertensive crisis.
Treatment of Hypertensive Crisis: Therapy should be interrupted with symptoms that may be prodromal or a manifestation of a hypertensive crisis, such as palpitations or headaches, and patients should be evaluated immediately. Discontinue tranylcypromine sulfate tablets, other drugs, foods or beverages suspected to contribute to the hypertensive crisis immediately [see Drug Interactions (7.1, 7.2)].
Patients with severe elevations in blood pressure (e.g., more than 180/120 mm Hg) with evidence of organ dysfunction require immediate blood pressure reduction. Fever should be managed by means of external cooling. However, additional measures to control the causes of hyperthermia (psychomotor agitation, increased neuromuscular activity, persistent seizures) may be required.
Hypertension
Clinically significant increases in blood pressure have also been reported
after the administration of MAOIs, including tranylcypromine sulfate tablets,
in patients not ingesting tyramine-rich foods or beverages. Assess blood
pressure before prescribing tranylcypromine sulfate tablets and closely
monitor blood pressure in all patients taking tranylcypromine sulfate tablets.
5.3 Serotonin Syndrome
The development of a potentially life-threatening serotonin syndrome has been reported with MAOIs when used concomitantly with other serotonergic drugs. Such drugs include SSRIs, SNRIs, tricyclic antidepressants, triptans, fentanyl, lithium, tramadol, tryptophan, buspirone, St. John’s wort, S-adenosyl-L-methionine (SAM-e), and other MAOIs used to treat nonpsychiatric disorders (such as linezolid or intravenous methylene blue).
Manifestations of the serotonin syndrome may include mental status changes (e.g., agitation, hallucinations, delirium, coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia; with possible rapid fluctuations of vital signs), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyper-reflexia, incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Fatal outcome of serotonin syndrome has been reported, including in patients who had been treated with tranylcypromine sulfate tablets. In some cases of an interaction between tranylcypromine sulfate tablets and SSRIs or SNRIs, the features of the syndrome resembled neuroleptic malignant syndrome.
The concomitant use, or use in rapid succession, of tranylcypromine sulfate tablets with other serotonergic drugs is contraindicated. However, there may be circumstances when treatment with other serotonergic substances (such as linezolid or intravenous methylene blue) is necessary and cannot be delayed. In such cases, tranylcypromine sulfate tablets must be discontinued as soon as possible before initiating treatment with the other agent.
Treatment with tranylcypromine sulfate tablets and any concomitant serotonergic agents should be discontinued immediately if the above events occur, and supportive symptomatic treatment should be initiated.
5.4 Activation of Mania or Hypomania
In patients with bipolar disorder, treating a depressive episode with tranylcypromine sulfate tablets or another antidepressant may precipitate a mixed/manic episode. Prior to initiating treatment with tranylcypromine sulfate tablets, screen patients for any personal or family history of bipolar disorder, mania, or hypomania.
5.5 Hypotension
Hypotension, including postural hypotension, has been observed during therapy with tranylcypromine sulfate tablets. At doses above 30 mg daily, postural hypotension is a major adverse reaction and may result in syncope. Symptoms of postural hypotension are seen most commonly, but not exclusively, in patients with pre-existing hypertension. Blood pressure usually returns rapidly to pretreatment levels upon discontinuation of tranylcypromine sulfate tablets.
Dosage increases should be made more gradually in patients with a tendency toward hypotension and/or postural hypotension (e.g., elderly patients) [see Dosage and Administration (2.2) and Use in Specific Populations (8.5)].Such patients should be closely observed for postural changes in blood pressure throughout treatment. Also, when tranylcypromine sulfate tablets are used concomitantly with other agents known to cause hypotension, the possibility of additive hypotensive effects should be considered [see Drug Interactions (7.1)].Postural hypotension may be relieved by having patients lie down until blood pressure returns to normal.
5.6 Hypotension and Hypertension during Anesthesia and Perioperative Care
It is recommended that tranylcypromine sulfate tablets be discontinued at least 10 days prior to elective surgery. If this is not possible, for general anesthesia, regional and local anesthesia, and perioperative care avoid the use of agents that are contraindicated for concomitant use with tranylcypromine sulfate tablets. Carefully consider the risk of agents and techniques that increase the risk for hypotension (e.g., epidural or spinal anesthesia) or other adverse reactions to tranylcypromine sulfate tablets (e.g., hypertension associated with the use of vasoconstrictors in local anesthetics).
5.7 Need for Emergency Treatment with Contraindicated Drugs
If in the absence of therapeutic alternatives emergency treatment with a contraindicated product (e.g., linezolid, intravenous methylene blue, direct- acting sympathomimetic drugs such as epinephrine) becomes necessary and cannot be delayed, discontinue tranylcypromine sulfate tablets as soon as possible before initiating treatment with the other product and monitor closely for adverse reactions [see Drug Interactions (7.1)].
5.8 Discontinuation Syndrome
Abrupt discontinuation or dosage reduction of tranylcypromine sulfate tablets has been associated with the appearance of new symptoms that include dizziness, nausea, headache, irritability, insomnia, diarrhea, anxiety, fatigue, abnormal dreams, and hyperhidrosis. In general, discontinuation events occurred more frequently with longer duration of therapy.
There have been spontaneous reports of adverse reactions occurring upon discontinuation of MAOIs, particularly when abrupt, including dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g. paresthesia, such as electric shock sensations), anxiety, confusion, headache, lethargy, emotional lability, insomnia, hypomania, tinnitus, and seizures. While these reactions are generally self-limiting, there have been reports of prolonged discontinuation symptoms.
Patients should be monitored for these symptoms when discontinuing treatment with tranylcypromine sulfate tablets. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible[see Dosage and Administration (2.3)and Adverse Reactions (6)].
5.9 Risk of Clinically Significant Adverse Reactions due to Persistence of
MAO Inhibition after Discontinuation
Although excretion of tranylcypromine sulfate tablet is rapid, inhibition of MAO may persist up to 10 days following discontinuation. This should be taken into account when considering the use of potentially interacting substances or the consumption of tyramine-rich food or beverages [see Drug Interactions (7.2)], or when interpreting adverse reactions observed after discontinuation of tranylcypromine sulfate tablets. Care should be taken to differentiate symptoms of persistent MAO inhibition from withdrawal symptoms [see Drug Abuse and Dependence (9.3)].
5.10 Hepatotoxicity
Hepatitis and elevated aminotransferases have been reported in association with tranylcypromine sulfate tablets administration. Patients should be monitored accordingly. Tranylcypromine sulfate tablets should be discontinued in patients who develop signs and symptoms of hepatotoxicity.
Sedation has occurred in tranylcypromine sulfate tablets-treated patients with cirrhosis. Patients with cirrhosis receiving tranylcypromine sulfate tablets should be monitored for possible increased risks of central nervous system adverse reactions, such as excessive drowsiness.
5.11 Seizures
Seizures have been reported with tranylcypromine sulfate tablets withdrawal after abuse, and with overdose. Patients at risk for seizures should be monitored accordingly.
5.12 Hypoglycemia in Diabetic Patients
Some MAOIs have contributed to hypoglycemic episodes in diabetic patients receiving insulin or other blood-glucose-lowering agents. Monitor blood glucose in patients receiving both tranylcypromine sulfate tablets and blood- glucose-lowering agents. A reduction of the dosage of such agents may be necessary [see Drug Interactions (7.1 )]
5.13 Aggravation of Coexisting Symptoms of Depression
Tranylcypromine sulfate tablets may aggravate coexisting symptoms in depression, such as anxiety and agitation.
5.14 Adverse Effects on the Ability to Drive and Operate Machinery
Some tranylcypromine sulfate tablets adverse reactions (e.g., hypotension, faintness, drowsiness, confusion, disorientation) can impair a patient’s ability to operate machinery or use an automobile. Patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that tranylcypromine sulfate tablets therapy does not impair their ability to engage in such activities.
- Activation of Mania/Hypomania: May be precipitated by antidepressant treatment in patients with bipolar disorder. Screen patients prior to treatment (5.4)
- Hypotension (including syncope): Monitor patients and adjust tranylcypromine sulfate tablets dosage or concomitant medication as necessary (5.5)
- Hypotension and Hypertension during Anesthesia and Perioperative Care: If possible, discontinue tranylcypromine sulfate tablets prior to elective surgery (5.6)
- Hepatitis and Elevated Liver Enzymes: Monitor accordingly (5.10)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reactions are described in greater detail in other sections:
- Suicidal thoughts and behaviors [see Warnings and Precautions (5.1)]
- Hypertensive crisis and hypertension [see Warnings and Precautions (5.2)]
- Serotonin syndrome [see Warnings and Precautions (5.3)]
- Activation of mania/hypomania [see Warnings and Precautions (5.4)]
- Hypotension [see Warnings and Precautions (5.5)]
- Hypotension and hypertension during anesthesia and perioperative care [see Warnings and Precautions (5.6)]
- Discontinuation syndrome [see Warnings and Precautions (5.8)]
- Persistence of MAO inhibition after discontinuation [see Warnings and Precautions (5.9)]
- Hepatotoxicity [see Warnings and Precautions (5.10)]
- Seizures[see Warnings and Precautions (5.11)]
- Hypoglycemia in diabetic patients [see Warnings and Precautions (5.12)]
- Aggravation of coexisting symptoms of depression [see Warnings and Precautions (5.13)]
- Adverse effects on the ability to drive and operate machinery [see Warnings and Precautions (5.14)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Based on clinical trial data, the most common adverse reactions to tranylcypromine were dry mouth, dizziness, insomnia, sedation, and headache (>30%) and overexcitement, constipation, blurred vision, and tremor (>10%).
The following adverse reactions have been identified in clinical trials or during post approval use of tranylcypromine sulfate tablets:
Blood and lymphatic system disorders: agranulocytosis, leukopenia, thrombocytopenia, anemia
Endocrine disorders: impaired water excretion compatible with the syndrome of inappropriate secretion of antidiuretic hormone (SIADH)
Metabolism and nutrition disorders: significant anorexia, weight gain
Psychiatric disorders: excessive stimulation/overexcitement, manic symptoms/hypomania, agitation, insomnia, anxiety, confusion, disorientation, loss of libido
Nervous system disorders: dizziness, restlessness/akathisia, akinesia, ataxia, myoclonic jerks, tremor, hyper-reflexia, muscle spasm, paresthesia, numbness, memory loss, sedation, drowsiness, dysgeusia, headaches (without blood pressure elevation)
Eye disorders: blurred vision, nystagmus
Ear and labyrinth disorders: tinnitus
Cardiac disorders: tachycardia, palpitations
Vascular disorders: hypertensive crisis, hypertension, hypotension (including postural hypotension with syncope)
Gastrointestinal disorders: diarrhea, constipation, nausea, abdominal pain, dry mouth, fissuring in corner of mouth
Hepatobiliary disorders: hepatitis, elevated aminotransferases
Skin and subcutaneous tissue disorders: localized scleroderma, flare-up of cystic acne, urticaria, rash, alopecia, sweating
Renal and urinary disorders: urinary retention, urinary incontinence, urinary frequency
Reproductive system and breast disorders: impotence, delayed ejaculation
General disorders and administration site conditions: edema, chills, weakness, fatigue/lethargy
Most common adverse reactions (>10%) were dry mouth, dizziness, insomnia, sedation, headache, overexcitement, constipation, blurred vision, and tremor (6)
** To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals USA, Inc. at 1-888-838-2872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.**
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Clinically Significant Drug Interactions
Tables 3 and 4 lists drug classes and individual products, respectively, with a potential for interaction with tranylcypromine sulfate tablets, describes the predominant observed or anticipated risks, and provides advice on concomitant use. Given serious adverse reactions with multiple agents, patients should avoid taking over-the-counter medications or dietary supplements without prior consultation with a healthcare provider able to provide advice on the potential for interactions.
Time to Start Tranylcypromine Sulfate Tablets after Discontinuation of a Contraindicated Drug
For products that are contraindicated with tranylcypromine sulfate tablets, a time period of 4 to 5 half-lives of the other product or any active metabolite should elapse before starting treatment with tranylcypromine sulfate tablets. After stopping treatment with an MAO inhibitor antidepressant, a time period of at least 1 week or 4 to 5 half-lives of the other MAO inhibitor (whichever is longer) should elapse before starting treatment with tranylcypromine sulfate tablets because of the risk for clinically significant adverse reactions after discontinuation due to persistent MAO inhibition [see Dosage and Administration (2.2), Warnings and Precautions (5.9) ]. This period can be several weeks long (e.g., a minimum of 5 weeks for fluoxetine given fluoxetine's long half-life). Refer to the prescribing information of the contraindicated product for relevant information.
Time to Start Contraindicated Drug after Discontinuation of Tranylcypromine
Sulfate Tablets
The potential for interactions persists after discontinuation of
tranylcypromine sulfate tablets until MAO activity has sufficiently recovered.
Inhibition of MAO may persist up to 10 days following discontinuation [see Warnings and Precautions (5.9)] . After stopping tranylcypromine sulfate
tablets, at least 1 week should elapse before starting another MAOI (intended
to treat MDD) or other contraindicated antidepressants. Refer to the
prescribing information of any agent considered for subsequent use for
recommendations on the duration of a waiting period after discontinuation of a
MAO inhibitor.
If in the absence of therapeutic alternatives and emergency treatment with a contraindicated drug (e.g., linezolid, intravenous methylene blue, direct- acting sympathomimetic drugs such as epinephrine) becomes necessary and cannot be delayed, discontinue tranylcypromine sulfate tablets as soon as possible before initiating treatment with the other agent, and monitor closely for adverse reactions.
Table 3 Clinically Significant Drug Interactions with Drug Classes*
|
Product |
Clinical Comment on Concomitant Use****a |
Predominant Effect/Risk**[Hypertensive Reaction (HR)bor Serotonin Syndrome (SS)c]** |
|
Agents with blood pressure-reducing effects |
Use with cautiond |
Hypotensione |
|
Non-selective H1 receptor antagonists |
Contraindicateda |
Increased anticholinergic effects |
|
Beta-adrenergic blockers (see also agents or procedures with blood pressure- reducing effects) |
Use with the cautiond |
More pronounced bradycardia, postural hypotensione |
|
Blood glucose-lowering agents |
Dosage reduction of such agents may be necessary. Monitor blood glucose. |
Excessive reduction of blood glucose (additive effect)f |
|
CNS depressant agents (including opioids, alcohol, sedatives, hypnotics) |
Use with cautiond |
Increased CNS depression |
|
Dietary supplements containing sympathomimetics |
Contraindicateda | |
|
Antidepressants including but not limited to:
|
Contraindicateda |
SS for all antidepressants |
|
Amphetamines and methylphenidates and derivatives |
Contraindicateda |
HR |
|
Sympathomimetic drugs** |
Contraindicateda |
HR; Including risk of intracerebral hemorrhage |
|
Triptans |
Contraindicateda |
SS |
- Some drugs in these groups may also be listed in Table 4 below.
** Sympathomimetic drugs include amphetamines as well as cold, hay fever or weight-reducing products that contain vasoconstrictors such as pseudoephedrine, phenylephrine, and ephedrine)
a[See Contraindications (4.1)]; b[See Warnings and Precautions (5.2)]; c[ See Warnings and Precautions (5.3)] d If not otherwise specified in this table, consider avoiding concomitant use (see also information on medication-free intervals, use agent at the lowest appropriate dosage, monitor for effects of the interaction, advise the patient to report potential effects).
e [See Warnings and Precautions (5.5)]; f [See Warnings and Precautions (5.14)]; g[See Overdosage (10.1)]****
** Table 4: Clinically Significant Drug Interactions with Individual Products***
|
Product |
Clinical Comment on Concomitant Use****a |
Predominant Effect/Risk**[Hypertensive Reaction (HR)bor Serotonin Syndrome (SS)c]** |
|
Altretamine |
Use with cautiond |
Orthostatic hypotensione |
|
Buspirone |
Contraindicateda |
HR |
|
Carbamazepine |
Contraindicateda |
SS |
|
Chlorpromazine |
Use with cautiond |
Hypotensive effectse |
|
Cyclobenzaprine |
Contraindicateda |
SS |
|
Dextromethorphan |
Contraindicateda |
SS; Psychosis, bizarre behavior |
|
Dopamine |
Contraindicateda |
HR |
|
Droperidol |
Use with cautiond |
QT interval prolongation |
|
Entacapone |
Use with cautiond |
HR |
|
Fentanyl |
Use with cautiond |
SS |
|
Hydroxytryptophan |
Contraindicateda |
SS |
|
Levodopa |
Contraindicateda |
HR |
|
Lithium |
Use with cautiond |
SS |
|
Meperidine |
Contraindicateda |
SS |
|
Methadone |
Use with cautiond |
SS |
|
Methyldopa |
Contraindicateda |
HR |
|
Metoclopramide |
Use with cautiond |
HR/SS |
|
Mirtazapine |
Contraindicateda |
SS |
|
Oxcarbazepine |
Use with cautiond because of close structural relationship with tricyclic antidepressants |
SS |
|
Rasagiline |
Contraindicateda |
HR |
|
Reserpine |
Contraindicateda |
HR |
|
S-adenosyl-L-methionine (SAM-e) |
Contraindicateda |
SS |
|
Tapentadol |
Contraindicateda |
HR/SS |
|
Tetrabenazine |
Contraindicateda |
HR |
|
Tolcapone |
Use with cautiond |
HR |
|
Tramadol |
Use with cautiond |
SS; Increased seizure risk |
|
Tryptophan |
Contraindicateda |
SS |
*Some drugs in this table may also belong to groups listed in Table 3 above, and may be associated with additional interactions.
a[See Contraindications (4.1)] ;b[See Warnings and Precautions (5.3)]; c[See Warnings and Precautions (5.7)]
dIf not otherwise specified in this table, consider avoiding concomitant use
(see also information on medication-free intervals , use agent at the lowest
appropriate dose, monitor for effects of the interaction, advise the patient
to report potential effects, and be prepared to discontinue the agent and
treat effects of the interaction
e[See Warnings and Precautions (5.5)]
7.2 Tyramine-Containing Foods and Beverages
Tranylcypromine sulfate tablets inhibit intestinal MAO, which is responsible for the catabolism of tyramine in food and beverages. As a result of this inhibition, large amounts of tyramine may enter the systemic circulation and precipitate a sudden elevation in blood pressure or hypertensive crisis [see Warnings and Precautions (5.2)].Instruct tranylcypromine sulfate tablets- treated patients to avoid foods and beverages with significant tyramine content during treatment with tranylcypromine sulfate tablets or within 2 weeks of stopping treatment (see Table 5 for a list of food and beverages containing significant amounts of tyramine).
Table 5: Foods and Beverages with and without Significant Amounts of Tyramine
|
Class of Food or |
Tyramine-Rich Foods and****Beverages to Avoid |
Acceptable Foods and Drinks, Containing No or Little Tyramine |
|
Meat, Poultry, and Fish |
Air dried, aged and fermented meats, sausages and salamis (including cacciatore, hard salami and mortadella); pickled herring; and any spoiled or improperly stored meat, poultry, and fish (e.g., foods that have undergone changes in coloration, odor, or become moldy); spoiled or improperly stored animal livers |
Fresh meat, poultry, and fish, including fresh processed meats (e.g., lunch meats, hot dogs, breakfast sausage, and cooked sliced ham) |
|
Vegetables |
Broad bean pods (fava bean pods) |
All other vegetables |
|
Dairy |
Aged cheeses |
Processed cheeses, mozzarella, ricotta cheese, cottage cheese, and yogurt |
|
Beverages |
All varieties of tap beer and beers that have not been pasteurized so as to allow for ongoing fermentation and excessive amounts of caffeine. |
Concomitant use of alcohol with tranylcypromine sulfate tabletsis not recommended. (Bottled and canned beers and wines contain little or no tyramine.) |
|
Other |
Concentrated yeast extract (e.g., Marmite), sauerkraut, most soybean products
(including soy sauce and |
Brewer’s yeast, baker’s yeast, soy milk, commercial chain restaurant pizzas prepared with cheeses low in tyramine |
See Full Prescribing Information for a list of products, foods and beverages that can interact with tranylcypromine sulfate tablets (7)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE & ADMINISTRATION
2.1 Recommended Dosage
Tranylcypromine sulfate tablets are for oral use. The recommended dosage is 30 mg per day (in divided doses). If patients do not have an adequate response, increase the dosage in increments of 10 mg per day every 1 to 3 weeks to a maximum 30 mg twice daily (60 mg per day). Dosage increases should be made more gradually in patients at risk for hypotension (e.g., geriatric patients) [see Warnings and Precautions (5.5)].
2.2 Switching to or from Other Antidepressants
Switching from Contraindicated Antidepressants to Tranylcypromine Sulfate
Tablets
After stopping treatment with contraindicated antidepressants, a time period
of 4 to 5 half-lives of the other antidepressant or any active metabolite
should elapse before starting treatment with tranylcypromine sulfate tablets.
After stopping treatment with an MAO inhibitor antidepressant, a time period
of at least one week or 4 to 5 half-lives of the other MAO inhibitor
(whichever is longer) should elapse before starting treatment with
tranylcypromine sulfate tablets to reduce the risk of additive effects [see Contraindications (4.1)and Drug Interactions (7.1)].
Switching from Tranylcypromine Sulfate Tablets to Other MAOIs or
Contraindicated Antidepressants
After stopping tranylcypromine sulfate tablets treatment, at least one week
should elapse before starting another MAOI (intended to treat MDD) or other
contraindicated antidepressants.Refer to the prescribing information of the
subsequently used drug for product-specific advice on a medication-free
interval [see Contraindications (4.1)and Drug Interactions (7.1)].
2.3 Discontinuing Treatment
Withdrawal effects, including delirium, have been reported with abrupt discontinuation of tranylcypromine sulfate tablets therapy. Higher daily doses and longer duration of use appear to be associated with a higher risk of withdrawal effects. Consider discontinuing tranylcypromine sulfate tablets therapy by slow, gradual dosage reduction[see Warnings and Precautions (5.8) and Drug Abuse and Dependence (9.3)].
2.4 Screen for Bipolar Disorder and Elevated Blood Pressure Prior to
Starting Tranylcypromine Sulfate Tablets
Prior to initiating treatment with tranylcypromine sulfate tablets:
- Screen patients for a history of mania [see Warnings and Precautions(5.4)].
- Measure blood pressure [see Warnings and Precautions (5.2, 5.5)].
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS & STRENGTHS
Tablets containing tranylcypromine sulfate equivalent to 10 mg tranylcypromine are round, rose-red, film-coated, and debossed on one side with "PARNATE" and "SB."
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are limited published reports of placental infarction and congenital
anomalies in association with use of tranylcypromine sulfate tablets during
pregnancy; however, these reports may not adequately inform the presence or
absence of drug-associated risk with the use of tranylcypromine sulfate
tablets during pregnancy. In the U.S. general population, the background risk
of major birth defects and miscarriage in clinically recognized pregnancies is
2-4% and 15-20%, respectively. Animal embryo-fetal development studies were
not conducted with tranylcypromine; however, published animal reproduction
studies report placental transfer of tranylcypromine in rats and a dose-
dependent decrease in uterine blood flow in pregnant sheep. Advise pregnant
women of the potential risk to a fetus.
Clinical Considerations
Labor or Delivery
During labor and delivery, the potential for interactions between
tranylcypromine sulfate tablets and drugs or procedures (e.g., epidural
anesthesia) should be taken into account in women who have received
tranylcypromine sulfate tablets[see Warnings and Precautions (5.6)and Drug Interactions (7.1)].
8.2 Lactation
Risk Summary
Tranylcypromine is present in human milk. There is no available information on
the effects of tranylcypromine on milk production. There is no available
information on the effects of tranylcypromine on a breastfed child; however,
because of the potential for serious adverse reactions in a breastfed infant,
advise nursing women to discontinue breastfeeding during treatment with
tranylcypromine sulfate tablets.
8.4 Pediatric Use
Safety and effectiveness of tranylcypromine sulfate tablets in the pediatric population have not been established. All risks associated with the use of tranylcypromine sulfate tablets, including the risk of suicidal thoughts and behavior, apply to adults and pediatric patients[see Boxed Warningand Warnings and Precautions (5)].
8.5 Geriatric Use
Older patients may be at greater risk of postural hypotension and other serious adverse reactions [see Warnings and Precautions (5)]. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
DRUG ABUSE AND DEPENDENCE SECTION
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
Abuse of tranylcypromine sulfate tablets has been reported. Some of these patients had a history of previous substance abuse.
The potential for abuse and the increased risk of serious adverse reactions with higher doses should be taken into account when considering the use of tranylcypromine sulfate tablets for patients at increased risk for substance abuse.
9.3 Dependence
Dependence, evidenced by precipitation of withdrawal effects following abrupt discontinuation of tranylcypromine sulfate tablets has been reported. Reported withdrawal effects included delirium (even with low daily doses), restlessness, anxiety, confusion, hallucinations, headache, weakness, diarrhea, and/or rapid relapse into depression. Thrombocytopenia and liver enzyme increases have also been observed in association with tranylcypromine sulfate tablets withdrawal from high doses [see Overdosage (10.1)]
Withdrawal effects have appeared within 1 to 3 days of discontinuation and have persisted for several weeks after discontinuation. The use of daily doses greater than recommended and longer duration of use appear to be associated with a higher risk of withdrawal effects.
Monitor for withdrawal effects for at least 1 week after discontinuation. Consider discontinuing tranylcypromine sulfate tablet therapy by slow, gradual dose reduction [see Dosage and Administration (2.3)] .
OVERDOSAGE SECTION
10 OVERDOSAGE
10.1 Overdosage Symptoms, Signs and Laboratory Abnormalities
Overdose of tranylcypromine sulfate tablets can cause the adverse reactions generally associated with tranylcypromine sulfate tablets administration [see Warnings and Precautions (5), Adverse Reactions (6)and Drug Interactions (7.1)]. However, these reactions may be more severe, including fatal reactions. Effects reported with overdosage of tranylcypromine sulfate tablets and/or other MAOIs include:
- Insomnia, restlessness, and anxiety, progressing in severe cases to agitation, mental confusion, and incoherence; delirium; seizures
- Hypotension, dizziness, weakness, and drowsiness, progressing in severe cases to extreme dizziness and shock
- Hypertension with severe headache and other symptoms/complications
- Twitching or myoclonic fibrillation of skeletal muscles, with hyperpyrexia, sometimes progressing to generalized rigidity and coma
10.2 Overdosage Management
There are no specific antidotes for tranylcypromine sulfate tablets. For current information on the management of poisoning or overdosage, contact a poison control center at 1-800-222-1222.
Abrupt withdrawal of tranylcypromine sulfate tablets following overdosage can precipitate withdrawal symptoms, including delirium [see Warnings and Precautions (5.9) and Drug Abuse and Dependence (9.3)].
Medical management should normally consist of general supportive measures, close observation of vital signs, and steps to counteract specific manifestations as they occur[see Warnings and Precautions (5)]. The toxic effects of tranylcypromine sulfate tablets may be delayed or prolonged following the last dose of the drug [see Clinical Pharmacology (12.2)]. Therefore, the patient should be closely observed for at least 1 week.
Data on the dialyzability of tranylcypromine are lacking.
DESCRIPTION SECTION
11 DESCRIPTION
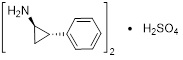
Tranylcypromine sulfate, the active ingredient of tranylcypromine sulfate tablets, is a non-hydrazine MAOI. The chemical name is (±)‑trans‑2‑phenylcyclopropylamine sulfate (2:1). The molecular formula is (C9H11N)2•H2SO4 and its molecular weight is 364.46. The structural formula is:
Tranylcypromine sulfate tablets film-coated tablets are intended for oral administration. Each round, rose‑red tablet is debossed on one side with the product name “PARNATE” and “SB” and contains tranylcypromine sulfate equivalent to 10 mg of tranylcypromine.
Inactive ingredients consist of microcrystalline cellulose, anhydrous citric acid, croscarmellose sodium, D&C Red No. 7, FD&C Blue No. 2, FD&C Yellow No. 6, gelatin, lactose, magnesium stearate, talc, titanium dioxide, carnauba wax, polyethylene glycol 400 and 8000, and hypromellose.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of tranylcypromine sulfate tablets as an antidepressant is not fully understood, but is presumed to be linked to potentiation of monoamine neurotransmitter activity in the central nervous system (CNS) resulting from its irreversible inhibition of the enzyme monoamine oxidase (MAO).
12.2 Pharmacodynamics
Although tranylcypromine is eliminated in 24 hours, recovery MAO activity takes up to 3 to 5 days[see Warnings and Precautions (5.9)].
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
No carcinogenesis, mutagenesis, or fertility impairment studies were conducted.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Tranylcypromine sulfate tablets are available as:
- 10 mg: film-coated, round, rose-red and debossed with the product name “PARNATE” and “SB” on one side of the tablet containing tranylcypromine sulfate equivalent to 10 mg of tranylcypromine.
- Bottles of 100 tablets: NDC 0591-5590-01
Store between 15° and 30°C (59° and 86°F). Dispense in a tight, light resistant container.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read FDA-approved patient labeling (Medication Guide).
Suicidal Thoughts and Behaviors
Advise patients and caregivers to look for the emergence of suicidal thoughts
and behaviors, especially early during treatment and when the dosage is
adjusted up or down [see Box Warningand Warnings and Precautions (5.1)].
Hypertensive Crisis
Advise the patient on possible symptoms and instruct the patient to seek
immediate medical attention if related signs or symptoms are present [see Boxed Warningand Warnings and Precautions (5.2)]
Serotonin Syndrome
Advise the patient on possible symptoms, and explain the potentially fatal
nature of serotonin syndrome and that it may result from an interaction with
other serotonergic drugs. Instruct the patient to seek immediate medical
attention if related signs or symptoms are present [see Warnings and Precautions (5.3)]
Interaction with Other Drugs and Dietary Supplements[see Contraindications (4.1)and Drug Interactions (7.1)]
- Warn the patient not to take concomitant medications, whether prescription or over the counter drugs, or dietary supplements without prior consultation with a health care provider able to provide advice on the potential for interactions.
- Explain to the patient that some other drugs may require a medication-free interval even after discontinuation of tranylcypromine sulfate tablets.
- Advise the patient to inform other physicians, pharmacists, and dentists about the treatment with tranylcypromine sulfate tablets.
Interaction with Foods and Beverages [see Contraindications (4.1) and Drug Interactions (7.2)]
- Warn the patient to avoid tyramine-rich foods and beverages.
- Advise the patient to avoid eating foods if storage conditions or freshness is unknown and to be cautious of foods of unknown age or composition even if refrigerated.
Hypotension
Advise the patient to report any symptoms of hypotension in the initial phase
of treatment to the healthcare provider, because occurrence of such symptoms
may require discontinuation [see Dosage and Administration (2.1) and Warnings and Precautions (5.5)].
Withdrawal Symptoms
Warn the patient not to stop tranylcypromine sulfate tablet treatment
abruptly, as withdrawal symptoms may occur and that the effect of
tranylcypromine sulfate tablets may continue even after discontinuation [see Warnings and Precautions (5.8, 5.9)].
Aggravation of Coexisting Symptoms of Depression
Inform the patient that tranylcypromine sulfate tablets may aggravate
coexisting symptoms in depression, such as anxiety and agitation and instruct
them to contact their healthcare provider if they experience such symptoms
[see Warnings and Precautions (5.13)].
Effects on Ability to Drive or Use Machinery[see Warnings and Precautions (5.14)]
- Warn the patient about the possible adverse reactions that can impair the performance of potentially hazardous tasks such as driving a car or operating machinery.
- Tell the patient not to operate hazardous machinery and automobiles until they are reasonably certain that their ability to engage in such activities is not impaired.
Made in Italy
Manufactured for:
Teva Pharmaceuticals USA, Inc.
Parsippany, NJ 07054
SPL MEDGUIDE SECTION
MEDICATION GUIDE
|
MEDICATION GUIDE |
What is the most important information I should know about tranylcypromine
sulfate tablets?
** Tranylcypromine sulfate tabletscan cause serious side effects including:**
Increase in suicidal thoughts or actionsin some children, teenagers, and young adults within the first few months of treatment and when the tranylcypromine sulfate tablet dose is changed. Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions.These include people who have, or have a family history of, bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.* Tranylcypromine sulfate tablets are not for use in children.**
How can I watch for and try to prevent suicidal thoughts and actions?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms
Call a healthcare provider right away if you have any of the following symptoms, especially if they are new, worse, or worry you:
|
|
|
|
|
|
|
|
|
|
|
|
*A sudden, severe increase in blood pressure (hypertensive crisis). A hypertensive crisis can happen when you eat certain foods and drinks certain beverages during or after tranylcypromine sulfate tablets treatment. A hypertensive crisis can lead to stroke and death. People who have thyroid problems (hyperthyroidism) may have a higher chance of having a hypertensive crisis. Symptoms of a hypertensive crisis may include:
|
|
|
|
|
|
|
|
|
|
A hypertensive crisis can also happen if you take tranylcypromine sulfate
tablets with certain other medicines. See, “Who should not take
tranylcypromine sulfate tablets?”
Avoid foods and drinks with a lot of tyramine while taking tranylcypromine
sulfate tabletsand for 2 weeks after you stop taking it. For a list of some
of the foods and drinks you should avoid during treatment with tranylcypromine
sulfate tablets see, “What should I avoid while taking tranylcypromine sulfate
tablets?”
What are tranylcypromine sulfate tablets?
****Tranylcypromine sulfate tablets are a prescription medicine used to treat
adults with a certain type of depression called major depressive disorder
(MDD) who have not responded well to treatment with other medicines used to
treat depression (antidepressants). Tranylcypromine sulfate tablets belong to
a class of medicines called monoamine oxidase inhibitors (MAOIs)
- It is important to talk with your healthcare provider about the risks of treating depression and the risk of not treating it. Talk with your healthcare provider about all your treatment choices.
- Tranylcypromine sulfate tablets are not for use as the first medicine to treat MDD.
- It is not known if tranylcypromine sulfate tablets are safe and effective for use in children.
Who should not take tranylcypromine sulfate tablets?
** Taking tranylcypromine sulfate tablets with certain antidepressants and
certain pain, allergy symptom, and cold and cough symptom medicines may cause
a potentially life-threatening hypertensive crisis or a problem called
serotonin syndrome.**See, “What is the most important information I should
know about tranylcypromine sulfate tablets?” and “What are the possible side
effects of tranylcypromine sulfate tablets?”
Do not take tranylcypromine sulfate tablets if you:
*take certain medicines, including: * antidepressants, such as: * other monoamine oxidase inhibitors (MAOIs) * selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs) * tricyclic antidepressants * other antidepressants, such as amoxapine, bupropion, maprotiline, nefazodone, trazodone, vilazodone, vortioxetine * amphetamines and methylphenidates * medicines that can raise blood pressure (sympathomimetic medicine), such as pseudoephedrine, phenylephrine and ephedrine. These medicines are in some cold, hay fever or weight-loss medicines. * sympathomimetic herbal medicines or dietary supplements * antihistamines (allergy medicines) * triptans * buspirone * carbamazepine * dextromethorphan * dopamine * hydroxytryptophan and tryptophan * levodopa and methyldopa * meperidine * rasagline * resperine * s-adenosyl-L-methionine (SAM-e) * tapentadol * tetrabenazin
Ask your healthcare provider or pharmacist if you are not sure if you take any of these medicines.
*have a tumor on your adrenal gland called a pheochromocytoma or a type of tumor called a paraganglioma.
Before taking tranylcypromine sulfate tablets, tell your healthcare provider about all your medical conditions, including if you:
- have high or low blood pressure
- have heart problems
- have cerebrovascular problems or have had a stroke
- have headaches
- have, or have a family history of, bipolar disorder, mania, or hypomania
- plan to have surgery
- have liver or thyroid problems
- have or have had seizures or convulsions
- have diabetes
- are pregnant or plan to become pregnant. Tranylcypromine sulfate tablets may harm your unborn baby.
- are breastfeeding or plan to breastfeed. Tranylcypromine sulfate passes into your breast milk. Do not breastfeed during treatment tranylcypromine sulfate tablets. Talk to your healthcare provider about the best way to feed your baby while taking tranylcypromine sulfate tablets.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Tranylcypromine sulfate tabletsand some other medicines may affect each other causing serious side effects. Tranylcypromine sulfate tabletsmay affect the way other medicines work, and other medicines may affect how tranylcypromine sulfate tablets work.
Some medicines need to be stopped for a period of time before you can start taking tranylcypromine sulfate tablets and for a period of time after you stop taking tranylcypromine sulfate tablets .
****Know the medicines you take. Keep a list of them to show your healthcare providers, pharmacist, and dentist when you get a new medicine.
How should I take tranylcypromine sulfate tablets?
- Take tranylcypromine sulfate tablets exactly as your healthcare provider tells you to take it.
- Your healthcare provider may need to change your dose of tranylcypromine sulfate tablets until it is the right dose for you.
- Do not stop taking tranylcypromine sulfate tablets without first talking to your healthcare provider. Stopping tranylcypromine sulfate tablets suddenly may cause withdrawal symptoms. See, “What are the possible side effects of tranylcypromine sulfate tablets?”
- Tell your healthcare provider if you think your condition has gotten worse during treatment with tranylcypromine sulfate tablets.
- If you take too many tranylcypromine sulfate tablets (overdose) call your healthcare provider or poison control, or go to the nearest hospital emergency room right away.
What should I avoid while taking tranylcypromine sulfate tablets?
- Do not eat foods or have drinks that have high amounts of tyramine while taking tranylcypromine sulfate tabletsor for 2 weeks after you stop taking tranylcypromine sulfate tablets.
- All foods you eat should be fresh or properly frozen.
- Avoid foods when you do not know how those foods should be stored.
- Ask your healthcare provider if you are not sure if certain foods and drinks contain tyramine.
The table below lists some of the foods and drinks you should avoid while you take tranylcypromine sulfate tablets.
|
Type of Food and Drink that contain Tyramine | |
|
Meat, Poultry, and Fish |
|
|
Vegetables |
|
|
Dairy (milk products) |
|
|
Drinks |
|
|
Other |
|
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how tranylcypromine sulfate tablets affects you.
- Do not drink alcohol while taking tranylcypromine sulfate tablets.
What are the possible side effects of tranylcypromine sulfate tablets?
****Tranylcypromine sulfate tablets may cause serious side effects,
including:
*See “What is the most important information I should know about tranylcypromine sulfate tablets?” *Serotonin Syndrome. A potentially life-threatening problem called serotonin syndrome can happen when you take tranylcypromine sulfate tabletswith certain other medicines. See, "Who should not take tranylcypromine sulfate tablets ?" Symptoms of serotonin syndrome may include:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
*Mania or hypomania (manic episodes) in people who have a history of bipolar disorder.
|
|
|
|
|
|
|
*Low blood pressure (hypotension) including a drop in your blood pressure when you stand or sit up (postural hypotension). This can happen more often in people who have high blood pressure (hypertension) and when the tranylcypromine sulfate tablet dose is changed. Postural hypotension may cause you to feel dizzy and faint (syncope). *Changes in your blood pressure (hypotension or hypertension) during surgery and during the time around surgery (perioperative). Taking tranylcypromine sulfate tablets with certain medicines used for anesthesia can cause hypotension or hypertension. If you plan to have surgery, tell your surgeon or the healthcare provider who will give you anesthesia that you take tranylcypromine sulfate tablet . Your healthcare provider should stop tranylcypromine sulfate tablets at least 10 days before you have surgery.
Withdrawal symptoms. Talk with your healthcare provider before you stop taking tranylcypromine sulfate tablets . Symptoms of withdrawal may include
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
*Liver problems *Seizures (convulsions). Seizures have happened in people who take too much tranylcypromine sulfate tablets . *Low blood sugar (hypoglycemia). Hypoglycemia has happened in people with diabetes who take medicines to lower blood sugar. Talk with your healthcare provider about checking your blood sugar during treatment with tranylcypromine sulfate tablets . Tell your healthcare provider if your blood sugar gets low. *Worsening of symptoms that can happen with depression, such as anxiety and agitation.
|
|
|
|
|
|
|
|
|
These are not all the side effects of tranylcypromine sulfate tablets .
Call your doctor for medical advice about side effects. You may report side
effects to FDA at 1-800-FDA-1088.
How do I store tranylcypromine sulfate tablets?
- Store tranylcypromine sulfate tablets between 59°F to 86°F (15°C to 30°C).
- Store tranylcypromine sulfate tablets in a tight, light resistant container.
Keep tranylcypromine sulfate tablets and all medicines out of the reach of children.
General information about the safe and effective use of tranylcypromine
sulfate tablets.
****Medicines are sometimes prescribed for purposes other than those listed
in a Medication Guide. Do not take tranylcypromine sulfate tablets for a
condition for which they were not prescribed. Do not give tranylcypromine
sulfate tablets to other people, even if they have the same symptoms you have.
It may harm them. You can ask your healthcare provider or pharmacist for
information about tranylcypromine sulfate tablets that is written for health
professionals.
What are the ingredients in tranylcypromine sulfate tablets?
** Active Ingredient:** tranylcypromine sulfate
Inactive Ingredients: microcrystalline cellulose, anhydrous citric acid,
croscarmellose sodium, D&C Red No. 7, FD&C Blue No. 2, FD&C Yellow No. 6,
gelatin, lactose, magnesium stearate, talc, titanium dioxide, carnauba wax,
polyethylene glycol 400 and 8000 and hypromellose
Made in Italy
Manufactured for:
Teva Pharmaceuticals USA, Inc.
Parsippany, NJ 07054
For more information, contact Teva at 1-888-838-2872.
This Medication Guide has been approved by the U.S. Food and Drug
Administration
Revised:3/2021
