Upside Down Pain Relief
31c2cd25-1fec-5ec5-e063-6394a90a24b4
HUMAN OTC DRUG LABEL
Aug 15, 2025
Fridababy, LLC
DUNS: 783729598
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Benzocaine, Glycerin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Upside Sown Pain Relief Spray
3.5 oz 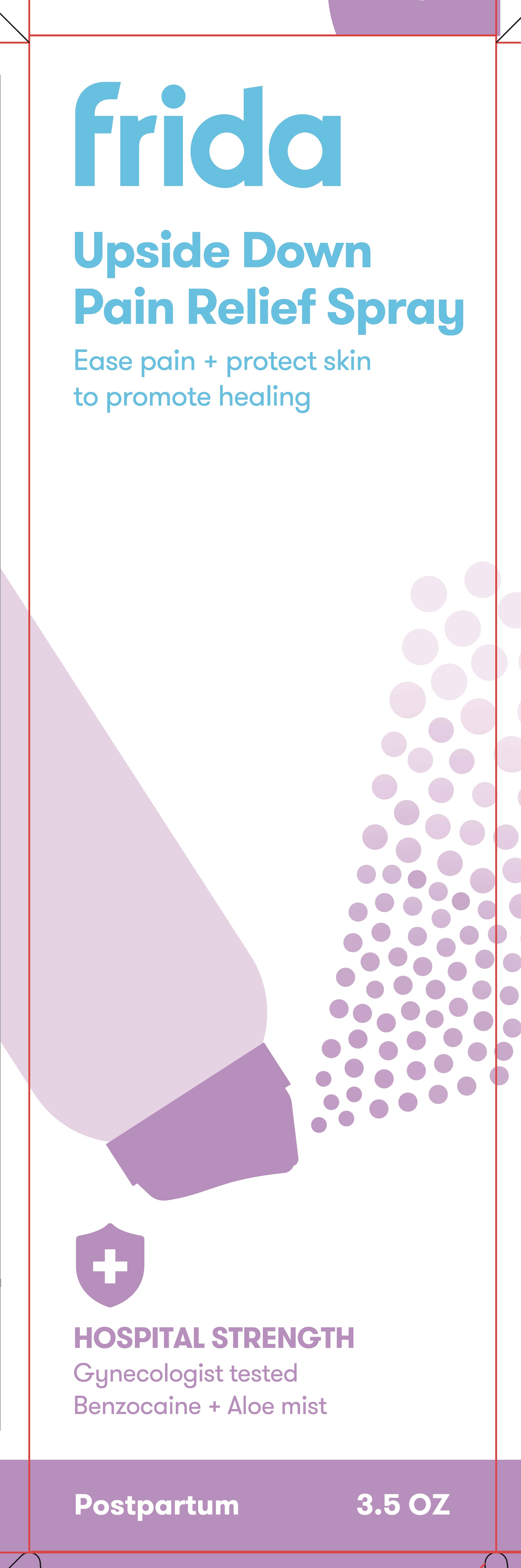
INDICATIONS & USAGE SECTION
Uses
■ Helps relieve discomfort
in the perianal area. ■ Temporarily protects
inflamed perianal skin.
DOSAGE & ADMINISTRATION SECTION
Directions
Adults: •When practical, cleanse the affected area with mild soap and warm water, and rinse thoroughly. •Gently pat dry before use. •Hold the can upside- down and point spray nozzle 6 to 12 inches from affected area. •Apply externally up to 6 times daily or after each bowel movement.
Children under 12 years of age: consult a doctor.
WARNINGS SECTION
Warnings
For extemal use only
FLAMMABLE. Do not use near heat,
flame, or fire or wlile smoking.
When using this product ■ Use only as
directed. ■ Do not exceed recommended
daily dosage unless directed by a doctor.
■ Intentional misuse by deliberately
concentrating or inhaling the contents
can be hmmful or fatal. ■ Do not put this
product into the rectum by using fingers
or any mechanical device or applicator.
Stop use and ask a doctor if
■ Condition worsens or does not improve
within 7 days. ■ Bleeding occurs
■ Certain persons can develop allergic
reactions to ingredients in this product. If the
symptoms being treated does not subside or if
redness, irritation, swelling, pain, or other
syptoms develop or increase, discontinue
use and consult a doctor. ■ Avoid spaying in
eyes. ■ Contents under pressure. Do not
puncture or incinerate. ■ Do not store at
temperature above 120ºF. Keep out of reach
of children. ■ If swallowed, get medical help
or contact a Poison Control Cerier right away.
Keep out of reach of children. •If swallowed, get medical help or contact a Poison Control Center right away.
INACTIVE INGREDIENT SECTION
Inactive Ingredients
aloe barbadensis leaf juice, citric acid, dimethyl ether, dipropylene glycol, ethanol, polyethylene glycol, potassium sorbate, PPG-3 methyl ether, sodium benzoate, witch hazel extract
OTC - PURPOSE SECTION
Purpose
Local anesthetic
Protectant
