Balmere Mineral Sunscreen Moisturizer Primer
Balmere Mineral Sunscreen Moisturizer + Primer
Approved
Approval ID
00d813e0-eb97-a6ba-e063-6394a90aa525
Product Type
HUMAN OTC DRUG LABEL
Effective Date
Aug 26, 2025
Manufacturers
FDA
Balmere
DUNS: 118839840
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Zinc Oxide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code83241-1039
Application NumberM020
Product Classification
M
Marketing Category
C200263
G
Generic Name
Zinc Oxide
Product Specifications
Route of AdministrationTOPICAL
Effective DateAugust 26, 2025
FDA Product Classification
INGREDIENTS (23)
LECITHIN, SOYBEANInactive
Code: 1DI56QDM62
Classification: IACT
POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATEInactive
Code: 9229XJ4V12
Classification: IACT
HYDROLYZED JOJOBA ESTERS (ACID FORM)Inactive
Code: UDR641JW8W
Classification: IACT
METHYLPROPANEDIOLInactive
Code: N8F53B3R4R
Classification: IACT
CAPRYLYL GLYCOLInactive
Code: 00YIU5438U
Classification: IACT
PHENYLPROPANOLInactive
Code: 0F897O3O4M
Classification: IACT
XANTHAN GUMInactive
Code: TTV12P4NEE
Classification: IACT
ISOAMYL LAURATEInactive
Code: M1SLX00M3M
Classification: IACT
HEXYLENE GLYCOLInactive
Code: KEH0A3F75J
Classification: IACT
TOCOPHEROLInactive
Code: R0ZB2556P8
Classification: IACT
CETYL ALCOHOLInactive
Code: 936JST6JCN
Classification: IACT
ETHYLHEXYLGLYCERINInactive
Code: 147D247K3P
Classification: IACT
GLYCERINInactive
Code: PDC6A3C0OX
Classification: IACT
SILICON DIOXIDEInactive
Code: ETJ7Z6XBU4
Classification: IACT
WATERInactive
Code: 059QF0KO0R
Classification: IACT
.ALPHA.-BISABOLOL, (+)-Inactive
Code: 105S6I733Z
Classification: IACT
BUTYLOCTYL SALICYLATEInactive
Code: 2EH13UN8D3
Classification: IACT
CETETH-20Inactive
Code: I835H2IHHX
Classification: IACT
PULLULANInactive
Code: 8ZQ0AYU1TT
Classification: IACT
SQUALANEInactive
Code: GW89575KF9
Classification: IACT
STEARETH-20Inactive
Code: L0Q8IK9E08
Classification: IACT
ZINC OXIDEActive
Quantity: 132 mg in 1 mL
Code: SOI2LOH54Z
Classification: ACTIB
CYCLODEXTRINSInactive
Code: 7E6SK9QDT8
Classification: IACT
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 5/20/2023
Balmere Mineral Sunscreen Moisturizer + Primer
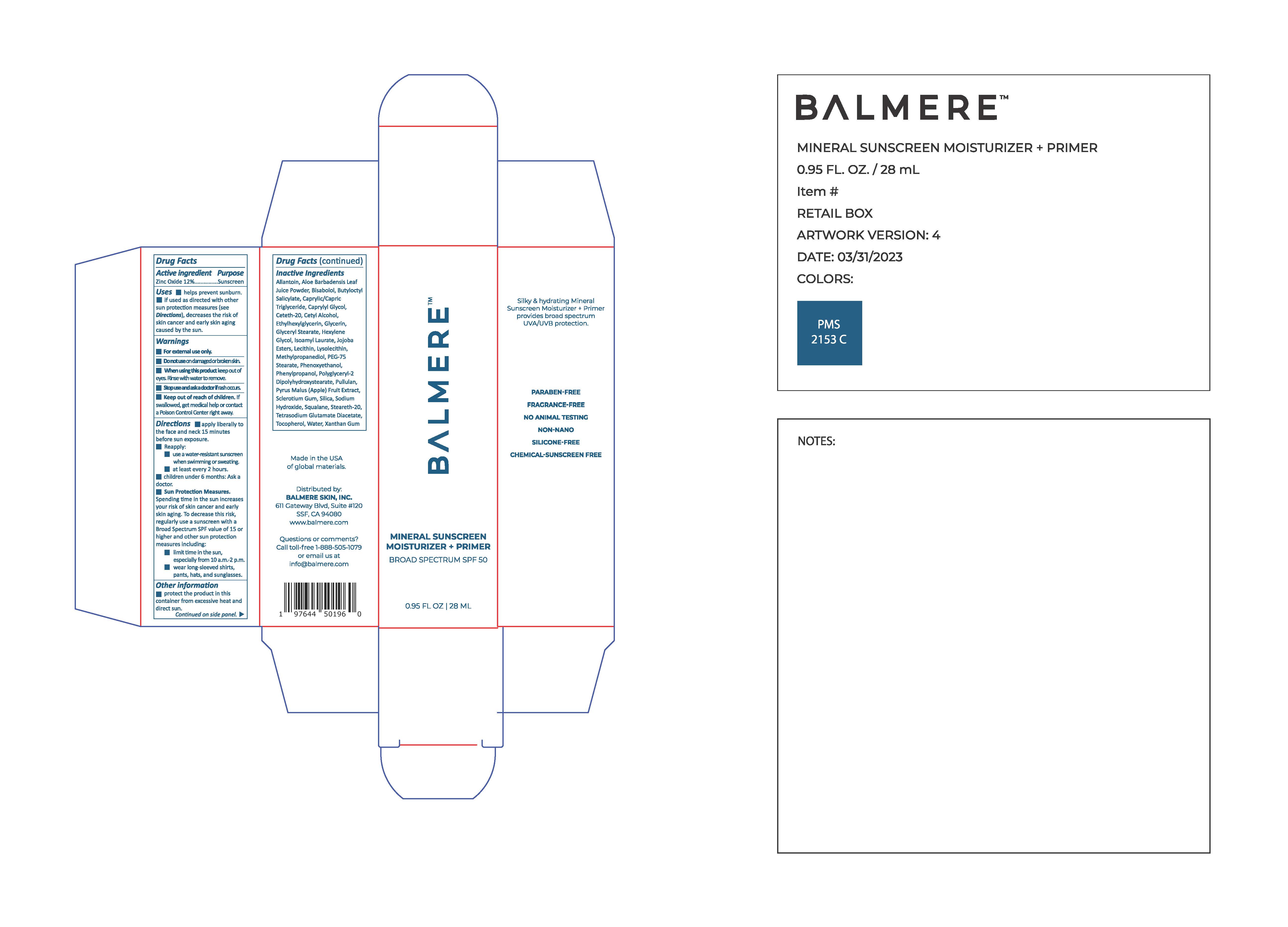
INDICATIONS & USAGE SECTION
LOINC: 34067-9Updated: 5/20/2023
Balmere Mineral Sunscreen Moisturizer + Primer

WARNINGS SECTION
LOINC: 34071-1Updated: 5/20/2023
Balmere Mineral Sunscreen Moisturizer + Primer

DOSAGE & ADMINISTRATION SECTION
LOINC: 34068-7Updated: 5/20/2023
Balmere Mineral Sunscreen Moisturizer + Primer

INACTIVE INGREDIENT SECTION
LOINC: 51727-6Updated: 5/20/2023
Balmere Mineral Sunscreen Moisturizer + Primer

OTC - ACTIVE INGREDIENT SECTION
LOINC: 55106-9Updated: 5/20/2023
Balmere Mineral Sunscreen Moisturizer + Primer

OTC - KEEP OUT OF REACH OF CHILDREN SECTION
LOINC: 50565-1Updated: 5/20/2023
Balmere Mineral Sunscreen Moisturizer + Primer

OTC - PURPOSE SECTION
LOINC: 55105-1Updated: 5/20/2023
Balmere Mineral Sunscreen Moisturizer + Primer

