CitraNatal 90 DHA
CitraNatal 90 DHA
81e639d7-352e-4eaa-87d9-582da68b9728
HUMAN PRESCRIPTION DRUG LABEL
Jan 8, 2024
Mission Pharmacal Company
DUNS: 008117095
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Vitamin C, Calcium, Iron, Vitamin D3, Vitamin E, Thiamin, Riboflavin, Niacinamide, Vitamin B6, Folic Acid, Iodine, Zinc, Copper, Docusate Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
CitraNatal ®90DHA Carton
NDC: 0178-0821-30
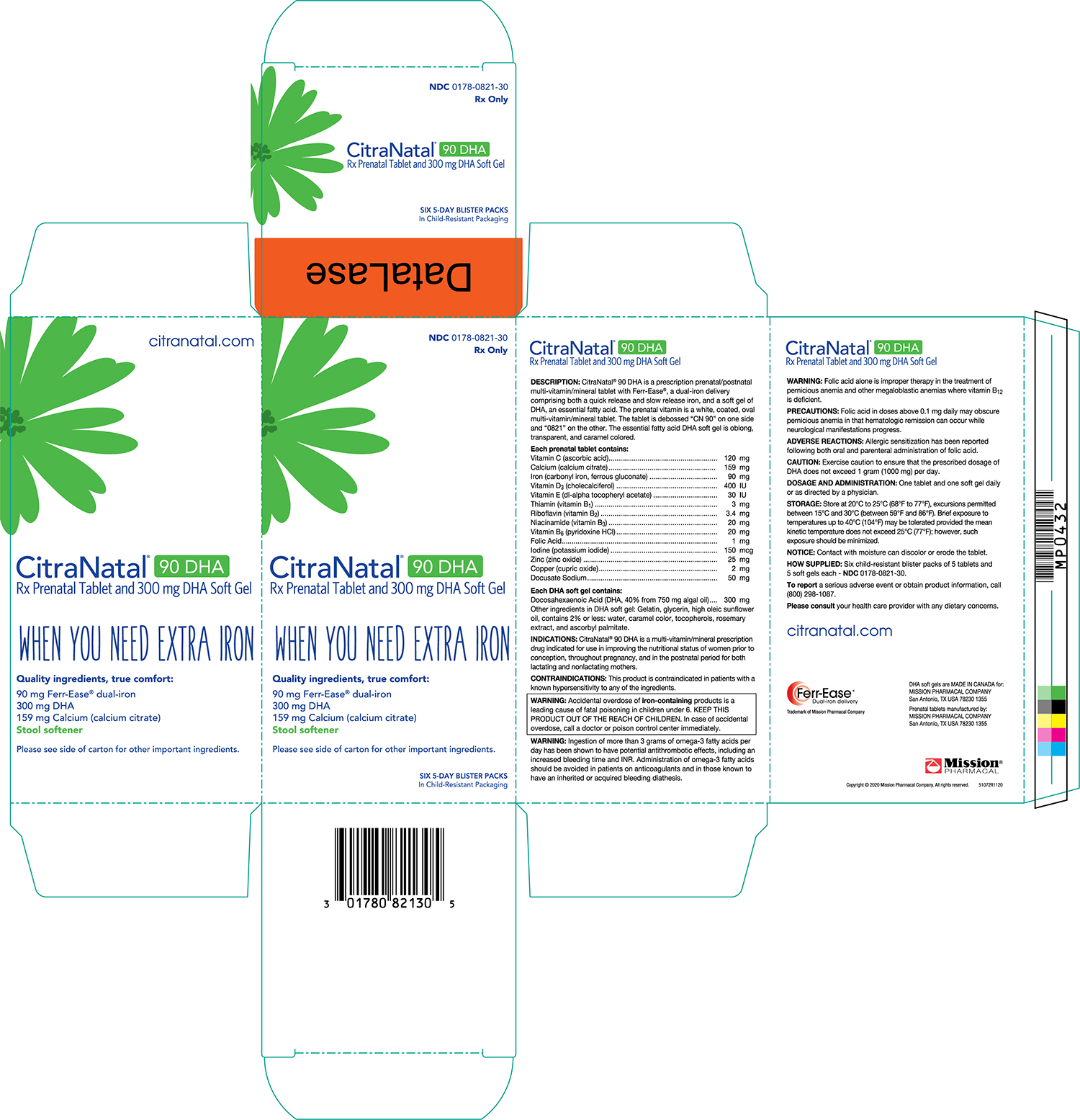
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
One tablet and one capsule daily or as directed by a physician.
STORAGE AND HANDLING SECTION
STORAGE
Store at 20-25°C (68-77°F)
**NOTICE:**Contact with moisture can discolor or erode the tablet.
HOW SUPPLIED
Six child-resistant blister packs of 5 tablets and 5 capsules each - NDC0178-0821-30
To reporta serious adverse event or obtain product information, call (210) 696-8400.
Please consultyour health care provider with any dietary concerns.
51072R1120
DHA capsules manufactured for:
MISSION PHARMACAL COMPANY
San Antonio, TX USA 78230 1355
Prenatal tablets manufactured by:
MISSION PHARMACAL COMPANY
San Antonio, TX USA 78230 1355
Ferr-Ease™
Dual-iron delivery
Trademark of Mission Pharmacal Company
