TETRACYCLINE HYDROCHLORIDE
TETRACYCLINE HYDROCHLORIDE CAPSULES, USP For Oral Use Rx only To reduce the development of drug-resistant bacteria and maintain the effectiveness of tetracycline hydrochloride and other antibacterial drugs, tetracycline hydrochloride should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
fb338af1-2b1b-413f-9653-10f318dbc4b3
HUMAN PRESCRIPTION DRUG LABEL
Oct 21, 2022
Strides Pharma Science Limited
DUNS: 650738743
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
TETRACYCLINE HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
TETRACYCLINE HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Tetracycline is a yellow, odorless, crystalline powder. Tetracycline is stable in air but exposure to strong sunlight causes it to darken. Its potency is affected in solutions of pH below 2 and is rapidly destroyed by alkali hydroxide solutions. Tetracycline is very slightly soluble in water, freely soluble in dilute acid and in alkali hydroxide solutions, sparingly soluble in alcohol, and practically insoluble in chloroform and in ether. The chemical name for tetracycline hydrochloride is 4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,-12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecar- boxamide monohydrochloride. Its structural formula is as follows:
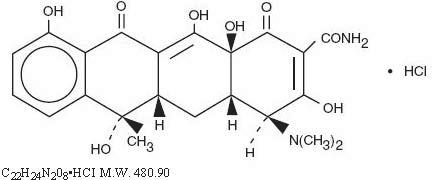
structural formula
Each capsule, for oral administration, contains 250 mg or 500 mg tetracycline hydrochloride.
**Inactive Ingredients:**Lactose Monohydrate, Colloidal Silicon Dioxide, Sodium Starch Glycolate, Pregelatinised Starch and Magnesium Stearate.
The 250 mg and 500 mg capsule shells contain D&C yellow no. 10, FD&C blue no. 1, FD&C yellow no. 6, gelatin, and titanium dioxide.
The imprinting ink for the 250 mg and 500 mg capsules contains Shellac, Black Iron Oxide and Potassium Hydroxide.
USP Dissolution Test 2.
