dAlba Vegan Fresh Sun Cushion
d'Alba Vegan Fresh Sun Cushion
Approved
Approval ID
20afcbab-a2f4-7965-e063-6294a90a83d1
Product Type
HUMAN OTC DRUG LABEL
Effective Date
Jun 8, 2025
Manufacturers
FDA
ADMTMAX LLC
DUNS: 101826554
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
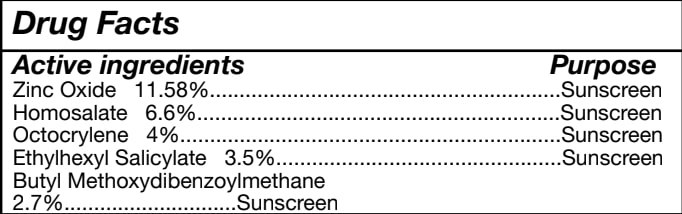
Zinc Oxide, Homosalate, Octocrylene
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code84662-060
Application NumberM020
Product Classification
M
Marketing Category
C200263
G
Generic Name
Zinc Oxide, Homosalate, Octocrylene
Product Specifications
Route of AdministrationTOPICAL
Effective DateJune 8, 2025
FDA Product Classification
INGREDIENTS (2)
GLYCERINInactive
Code: PDC6A3C0OX
Classification: IACT
ZINC OXIDEActive
Quantity: 11.58 mg in 25 g
Code: SOI2LOH54Z
Classification: ACTIB
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 8/27/2024

INDICATIONS & USAGE SECTION
LOINC: 34067-9Updated: 8/27/2024

DOSAGE & ADMINISTRATION SECTION
LOINC: 34068-7Updated: 8/27/2024

WARNINGS SECTION
LOINC: 34071-1Updated: 8/27/2024

INACTIVE INGREDIENT SECTION
LOINC: 51727-6Updated: 8/27/2024

OTC - KEEP OUT OF REACH OF CHILDREN SECTION
LOINC: 50565-1Updated: 8/27/2024

OTC - PURPOSE SECTION
LOINC: 55105-1Updated: 8/27/2024

OTC - ACTIVE INGREDIENT SECTION
LOINC: 55106-9Updated: 8/27/2024