Registrants (1)
079637826
Manufacturing Establishments (1)
Bionpharma Inc.
Bionpharma Inc.
780516717
Products (1)
Clobazam
69452-116
ANDA208819
ANDA (C73584)
ORAL
December 26, 2023
Drug Labeling Information
DESCRIPTION SECTION
11 DESCRIPTION
Table 4. Description|
Proprietary Name: |
None |
|
Established Name: |
Clobazam Oral Suspension |
|
Dosage Forms: |
Oral Suspension |
|
Route of Administration: |
Oral |
|
Established Pharmacologic Class of Drug: |
Benzodiazepine |
|
Chemical Name: |
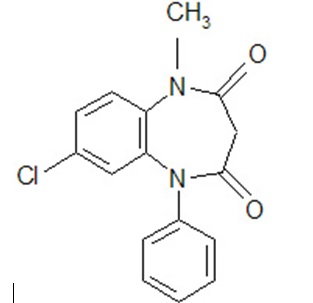
7-Chloro-1-methyl-5-phenyl-1H-1,5 benzodiazepine-2,4( 3H,5H)-dione |
|
Structural Formula: |
|
Clobazam is a white to almost white crystalline powder with a slightly bitter taste; is practically insoluble in water, sparingly soluble in ethanol and in methanol, freely soluble in dichloromethane and in acetone. The melting range of clobazam is from 182ºC to 185ºC. The molecular formula is C 16H 13O 2N 2Cl and the molecular weight is 300.7.
Clobazam is available for oral administration as a white to off-white suspension containing clobazam at a concentration of 2.5 mg/mL. Inactive ingredients include artificial mixed berry flavor, citric acid monohydrate, disodium hydrogen phosphate dihydrate, magnesium aluminum silicate, maltitol solution, methylparaben, polysorbate 80, propylene glycol, propylparaben, purified water, simethicone emulsion, sucralose powder, and xanthan gum.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The exact mechanism of action for clobazam, a 1,5-benzodiazepine, is not fully understood but is thought to involve potentiation of GABAergic neurotransmission resulting from binding at the benzodiazepine site of the GABA Areceptor.
12.2 Pharmacodynamics
Effects on Electrocardiogram
The effect of clobazam 20 mg and 80 mg administered twice daily on QTc interval was evaluated in a randomized, evaluator-blinded, placebo-, and active-controlled (moxifloxacin 400 mg) parallel thorough QT study in 280 healthy subjects. In a study with demonstrated ability to detect small effects, the upper bound of the one-sided 95% confidence interval for the largest placebo-adjusted, baseline-corrected QTc based on the Fridericia correction method was below 10 ms, the threshold for regulatory concern. Thus, at a dose two times the maximum recommended dose, clobazam did not prolong the QTc interval to any clinically relevant extent.
12.3 Pharmacokinetics
The peak plasma levels (C max) and the area under the curve (AUC) of clobazam are dose-proportional over the dose range of 10 mg to 80 mg following single- or multiple-dose administration of clobazam. Based on a population pharmacokinetic analysis, the pharmacokinetics of clobazam are linear from 5 mg/day to 160 mg/day. Clobazam is converted to N-desmethylclobazam which has about 1/5 the activity of clobazam. The estimated mean elimination half-lives (t 1/2) of clobazam and N-desmethylclobazam were 36 hours to 42 hours and 71 hours to 82 hours, respectively.
Absorption
Clobazam is rapidly and extensively absorbed following oral administration. The time to peak concentrations (T max) of clobazam tablets under fasted conditions ranged from 0.5 hour to 4 hours after single- or multiple-dose administrations. The relative bioavailability of clobazam tablets compared to an oral solution is approximately 100%. After single dose administration of the oral suspension under fasted conditions, the T maxranged from 0.5 hour to 2 hours. Based on exposure (C maxand AUC) of clobazam, clobazam tablets and suspension were shown to have similar bioavailability under fasted conditions. The administration of clobazam tablets with food or when crushed in applesauce does not affect absorption. Although not studied, the oral bioavailability of the oral suspension is unlikely to be affected under fed conditions.
Distribution
Clobazam is lipophilic and distributes rapidly throughout the body. The apparent volume of distribution at steady state was approximately 100 L. The in vitroplasma protein binding of clobazam and N-desmethylclobazam is approximately 80% to 90% and 70%, respectively.
Metabolism and Excretion
Clobazam is extensively metabolized in the liver, with approximately 2% of the dose recovered in urine and 1% in feces as unchanged drug. The major metabolic pathway of clobazam involves N-demethylation, primarily by CYP3A4 and to a lesser extent by CYP2C19 and CYP2B6. N-desmethylclobazam, an active metabolite, is the major circulating metabolite in humans, and at therapeutic doses, plasma concentrations are 3 times to 5 times higher than those of the parent compound. Based on animal and in vitroreceptor binding data, estimates of the relative potency of N-desmethylclobazam compared to parent compound range from 1/5 to equal potency. N-desmethylclobazam is extensively metabolized, mainly by CYP2C19. N-desmethylclobazam and its metabolites comprise ~94% of the total drug-related components in urine. Following a single oral dose of radiolabeled drug, approximately 11% of the dose was excreted in the feces and approximately 82% was excreted in the urine.
The polymorphic CYP2C19 is the major contributor to the metabolism of the pharmacologically active N-desmethylclobazam [see Clinical Pharmacology ( 12.5)] . In CYP2C19 poor metabolizers, levels of N-desmethylclobazam were 5-fold higher in plasma and 2- to 3-fold higher in the urine than in CYP2C19 extensive metabolizers.
Pharmacokinetics in Specific Populations
Age
Population pharmacokinetic analyses showed that the clearance of clobazam is lower in elderly subjects compared to other age groups (ages 2 to 64). Dosing should be adjusted in the elderly [see Dosage and Administration ( 2.4)] .
Sex
Population pharmacokinetic analyses showed no difference in the clearance of clobazam between women and men.
Race
Population pharmacokinetic analyses including Caucasian (75%), African American (15%), and Asian (9%) subjects showed that there is no evidence of clinically significant effect of race on the clearance of clobazam.
Renal Impairment
The effect of renal impairment on the pharmacokinetics of clobazam was evaluated in patients with mild (creatinine clearance [CL CR] > 50 mL/min to 80 mL/min; N = 6) and moderate (CL CR = 30 mL/min to 50 mL/min; N = 6) renal dysfunction, with matching healthy controls (N = 6), following administration of multiple doses of clobazam 20 mg/day. There were insignificant changes in C max(3% to 24%) and AUC (≤ 13%) for clobazam or N-desmethylclobazam in patients with mild or moderate renal impairment compared to patients with normal renal function. Patients with severe renal impairment or ESRD were not included in this study.
Hepatic Impairment
There are limited data to characterize the effect of hepatic impairment on the pharmacokinetics of clobazam. In a small study, the pharmacokinetics of a 20 mg single oral dose of clobazam in 9 patients with liver impairment were compared to healthy controls (N = 6). The C maxand the mean plasma clearance of clobazam, as well as the C maxof N-desmethylclobazam, showed no significant change compared to the healthy controls. The AUC values of N-desmethylclobazam in these patients were not available. Adjust dosage in patients with hepatic impairment [see Dosage and Administration ( 2.7)] .
Drug Interaction Studies
In vitro studies:
Clobazam did not inhibit CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP3A4, UGT1A1, UGT1A4, UGT1A6, or UGT2B4 in vitro. N-desmethylclobazam showed weak inhibition of CYP2C9, UGT1A4, UGT1A6 and UGT2B4.
Clobazam and N-desmethylclobazam did not significantly increase CYP1A2 or CYP2C19 activities, but did induce CYP3A4 activity in a concentration- dependent manner. Clobazam and N-desmethylclobazam also increased UGT1A1 mRNA but at concentrations much higher than therapeutic levels. The potential for clobazam or N-desmethylclobazam to induce CYP2B6 and CYP2C8 has not been evaluated.
Clobazam and N-desmethylclobazam do not inhibit P-glycoprotein (P-gp), but are P-gp substrates.
In vivo studies:
Potential for Clobazam to Affect Other Drugs
The effect of repeated 40 mg once-daily doses of clobazam on the pharmacokinetic profiles of single-dose dextromethorphan (CYP2D6 substrate), midazolam (CYP3A4 substrate), caffeine (CYP1A2 substrate), and tolbutamide (CYP2C9 substrate), was studied when these probe substrates were given as a drug cocktail (N = 18).
Clobazam increased AUC and C maxof dextromethorphan by 90% and 59%, respectively, reflecting its inhibition of CYP2D6 in vivo. Drugs metabolized by CYP2D6 may require dose adjustment when used with clobazam.
Clobazam decreased the AUC and C maxof midazolam by 27% and 24%, respectively, and increased the AUC and C maxof the metabolite 1-hydroxymidazolam by 4-fold and 2-fold, respectively. This level of induction does not call for dosage adjustment of drugs that are primarily metabolized by CYP3A4 when used concomitantly with clobazam. Some hormonal contraceptives are metabolized by CYP3A4 and their effectiveness may be diminished when given with clobazam [see Drug Interactions ( 7.3)] . Repeated clobazam doses had no effect on caffeine and tolbutamide.
A population pharmacokinetic analysis indicated clobazam did not affect the exposure of valproic acid (a CYP2C9/2C19 substrate) or lamotrigine (a UGT substrate).
Potential for Other Drugs to Affect Clobazam
Co-administration of ketoconazole (a strong CYP3A4 inhibitor) 400 mg once- daily for 5 days increased clobazam AUC by 54%, with an insignificant effect on clobazam C max. There was no significant change in AUC and C maxof N-desmethylclobazam (N = 18).
Strong (e.g., fluconazole, fluvoxamine, ticlopidine) and moderate (e.g., omeprazole) inhibitors of CYP2C19 may result in up to a 5-fold increase in exposure to N-desmethylclobazam, the active metabolite of clobazam, based on extrapolation from pharmacogenomic data [see Clinical Pharmacology ( 12.5)] . Dosage adjustment of clobazam may be necessary when co-administered with strong or moderate CYP2C19 inhibitors [see Drug Interactions ( 7.4)] .
The effects of concomitant antiepileptic drugs that are CYP3A4 inducers (phenobarbital, phenytoin, and carbamazepine), CYP2C19 inducers (valproic acid, phenobarbital, phenytoin, and carbamazepine), and CYP2C19 inhibitors (felbamate and oxcarbazepine) were evaluated using data from clinical trials. Results of population pharmacokinetic analysis show that these concomitant antiepileptic drugs did not significantly alter the pharmacokinetics of clobazam or N-desmethylclobazam at steady-state.
Alcohol has been reported to increase the maximum plasma exposure of clobazam by approximately 50%. Alcohol may have additive CNS depressant effects when taken with clobazam [see Warnings and Precautions ( 5.4), Drug Interactions ( 7.2)] .
12.5 Pharmacogenomics
The polymorphic CYP2C19 is the main enzyme that metabolizes the pharmacologically active N-desmethylclobazam. Compared to CYP2C19 extensive metabolizers, N-desmethylclobazam AUC and C maxare approximately 3 times to 5 times higher in poor metabolizers (e.g., subjects with *2/*2 genotype) and 2 times higher in intermediate metabolizers (e.g., subjects with *1/*2 genotype). The prevalence of CYP2C19 poor metabolism differs depending on racial/ethnic background. Dosage in patients who are known CYP2C19 poor metabolizers may need to be adjusted [see Dosage and Administration ( 2.5)] .
The systemic exposure of clobazam is similar for both CYP2C19 poor and extensive metabolizers.
DRUG INTERACTIONS SECTION
Highlight: * Alcohol: Increases blood levels of clobazam by about 50% ( 7.2)
- Drugs metabolized by CYP2D6: Lower doses of these drugs may be required when used concomitantly with clobazam ( 7.3)
- Strong or Moderate CYP2C19 Inhibitors: Dosage adjustment of clobazam may be necessary ( 7.4)
7 DRUG INTERACTIONS
7.1 Opioids
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABA Asites, and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Limit dosage and duration of concomitant use of benzodiazepines and opioids, and follow patients closely for respiratory depression and sedation [see Warnings and Precautions ( 5.1)] .
7.2 CNS Depressants and Alcohol
Concomitant use of clobazam with other CNS depressants may increase the risk of sedation and somnolence [see Warnings and Precautions ( 5.4)].
Alcohol, as a CNS depressant, will interact with clobazam in a similar way and also increases clobazam’s maximum plasma exposure by approximately 50%. Therefore, caution patients or their caregivers against simultaneous use with other CNS depressant drugs or alcohol, and caution that the effects of other CNS depressant drugs or alcohol may be potentiated [see Warnings and Precautions ( 5.4)] .
7.3 Effect of Clobazam on Other Drugs
Hormonal Contraceptives
Clobazam is a weak CYP3A4 inducer. As some hormonal contraceptives are metabolized by CYP3A4, their effectiveness may be diminished when given with clobazam. Additional non-hormonal forms of contraception are recommended when using clobazam [see Clinical Pharmacology ( 12.3), Patient Counseling Information ( 17)] .
Drugs Metabolized by CYP2D6
Clobazam inhibits CYP2D6. Dose adjustment of drugs metabolized by CYP2D6 may be necessary [see Clinical Pharmacology ( 12.3)] .
7.4 Effect of Other Drugs on Clobazam
Strong and moderate inhibitors of CYP2C19
Strong and moderate inhibitors of CYP2C19 may result in increased exposure to N-desmethylclobazam, the active metabolite of clobazam. This may increase the risk of dose-related adverse reactions. Dosage adjustment of clobazam may be necessary when co-administered with strong CYP2C19 inhibitors (e.g., fluconazole, fluvoxamine, ticlopidine) or moderate CYP2C19 inhibitors (e.g., omeprazole) [see Clinical Pharmacology ( 12.3)] .
OVERDOSAGE SECTION
10 OVERDOSAGE
Overdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion, dysarthria, lethargy, hypnotic state, diminished reflexes, ataxia, and hypotonia. Rarely, paradoxical or disinhibitory reactions (including agitation, irritability, impulsivity, violent behavior, confusion, restlessness, excitement, and talkativeness) may occur. In severe overdosage cases, patients may develop respiratory depression and coma. Overdosage of benzodiazepines in combination with other CNS depressants (including alcohol and opioids) may be fatal [see Warnings and Precautions ( 5.2)] . Markedly abnormal (lowered or elevated) blood pressure, heart rate, or respiratory rate raise the concern that additional drugs and/or alcohol are involved in the overdosage.
In managing benzodiazepine overdosage, employ general supportive measures, including intravenous fluids and airway maintenance. Flumazenil, a specific benzodiazepine receptor antagonist indicated for the complete or partial reversal of the sedative effects of benzodiazepines in the management of benzodiazepine overdosage, can lead to withdrawal and adverse reactions, including seizures, particularly in the context of mixed overdosage with drugs that increase seizure risk (e.g., tricyclic and tetracyclic antidepressants) and in patients with long-term benzodiazepine use and physical dependency. The risk of withdrawal seizures with flumazenil may be increased in patients with epilepsy. Flumazenil is contraindicated in patients who have received a benzodiazepine for control of a potentially life-threatening condition (e.g., status epilepticus). If the decision is made to use flumazenil, it should be used as an adjunct to, not as a substitute for, supportive management of benzodiazepine overdosage. See the flumazenil injection Prescribing Information.
Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In mice, oral administration of clobazam (0 mg/kg/day, 6 mg/kg/day, 12 mg/kg/day, or 24 mg/kg/day) for 2 years did not result in an increase in tumors. The highest dose tested was approximately 3 times the maximum recommended human dose (MRHD) of 40 mg/day, based on body surface area (mg/m 2).
In rats, oral administration of clobazam for 2 years resulted in increases in tumors of the thyroid gland (follicular cell adenoma and carcinoma) and liver (hepatocellular adenoma) at the mid and high doses. The low dose, not associated with an increase in tumors, was associated with plasma exposures (AUC) for clobazam and its major active metabolite, N-desmethylclobazam, less than that in humans at the MRHD.
Mutagenesis
Clobazam and the major active metabolite, N-desmethylclobazam, were negative for genotoxicity, based on data from a battery of in vitro(bacteria reverse mutation, mammalian clastogenicity) and in vivo(mouse micronucleus) assays.
Impairment of Fertility
In a fertility study in which clobazam (50 mg/kg/day, 350 mg/kg/day, or 750 mg/kg/day, corresponding to 12 times, 84 times and 181 times the oral Maximum Recommended Human Dose, MRHD, of 40 mg/day based on mg/m 2body surface) was orally administered to male and female rats prior to and during mating and continuing in females to gestation day 6, increases in abnormal sperm and pre- implantation loss were observed at the highest dose tested. The no-effect level for fertility and early embryonic development in rats was associated with plasma exposures (AUC) for clobazam and its major active metabolite, N-desmethylclobazam, less than those in humans at the maximum recommended human dose of 40 mg/day.
SPL MEDGUIDE SECTION
|
MEDICATION GUIDE **Clobazam (**KLOE-ba-zam) Oral Suspension, C-IV |
|
What is the most important information I should know about clobazam oral suspension? ***Clobazam oral suspension is a benzodiazepine medicine.****Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system (CNS) depressants (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma, and death.**Get emergency help right away if any of the following happens: * shallow or slowed breathing * breathing stops (which may lead to the heart stopping) * excessive sleepiness (sedation) Do not drive or operate heavy machinery until you know how taking clobazam oral suspension with opioids affects you. Risk of abuse, misuse, and addiction.**There is a risk of abuse, misuse, and addiction with benzodiazepines, including clobazam oral suspension, which can lead to overdose and serious side effects including coma and death. Serious side effects including coma and death have happened in people who have abused or misused benzodiazepines, including clobazam oral suspension.These serious side effects may also include delirium, paranoia, suicidal thoughts or actions, seizures, and difficulty breathing.Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these serious side effects. *You can develop an addiction even if you take clobazam oral suspension as prescribed by your healthcare provider. Take clobazam oral suspension exactly as your healthcare provider prescribed. * Do not share your clobazam oral suspension with other people. * Keep clobazam oral suspension in a safe place and away from children. Physical dependence and withdrawal reactions.Clobazam oral suspension can cause physical dependence and withdrawal reactions. Do not suddenly stop taking clobazam oral suspension.**Stopping clobazam oral suspension suddenly can cause serious and life-threatening side effects, including, unusual movements, responses, or expressions, seizures, sudden and severe mental or nervous system changes, depression, seeing or hearing things that others do not see or hear, an extreme increase in activity or talking, losing touch with reality, and suicidal thoughts or actions.Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these symptoms. *****Some people who suddenly stopbenzodiazepines********have symptoms that can last for several weeks to more than 12 months***, including, anxiety, trouble remembering, learning, or concentrating, depression, problems sleeping, feeling like insects are crawling under your skin, weakness, shaking, muscle twitching, burning or prickling feeling in your hands, arms, legs or feet, and ringing in your ears. * Physical dependence is not the same as drug addiction. Your healthcare provider can tell you more about the differences between physical dependence and drug addiction. * Do not take more clobazam oral suspension than prescribed or take clobazam oral suspension for longer than prescribed. *Clobazam oral suspension can make you sleepy or dizzy and can slow your thinking and motor skills. * Do not drive, operate heavy machinery, or do other dangerous activities until you know how clobazam oral suspension affects you. * Do not drink alcohol or take other drugs that may make you sleepy or dizzy while taking clobazam oral suspension without first talking to your healthcare provider. When taken with alcohol or drugs that cause sleepiness or dizziness, clobazam oral suspension may make your sleepiness or dizziness much worse. ***Serious skin reactions have been seen when clobazam oral suspension is taken with other medicines and may require stopping its use.**Do not stop taking clobazam oral suspension without first talking to your healthcare provider. * A serious skin reaction can happen at any time during your treatment with clobazam oral suspension, but is more likely to happen within the first 8 weeks of treatment. These skin reactions may need to be treated right away. * Call your healthcare provider immediately if you have skin blisters, rash, sores in the mouth, hives or any other allergic reaction. *Like other antiepileptic medicines, clobazam oral suspension may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. Call your healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
How can I watch for early symptoms of suicidal thoughts and actions?
Call your healthcare provider between visits as needed, especially if you are worried about symptoms. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus). Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes. |
|
What is****clobazam oral suspension?
It is not known if clobazam oral suspension is safe and effective in children less than 2 years old. |
|
Do not takeclobazam oral suspensionif you:
Before you take****clobazam oral suspension, tell your healthcare provider about all your medical conditions, including if you:
o Breastfeeding during treatment with clobazam oral suspension may cause your baby to have sleepiness, feeding problems, and decreased weight gain. o Talk to your healthcare provider about the best way to feed your baby if you take clobazam oral suspension. **Tell your healthcare provider about all the medicines you take,**including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking clobazam oral suspension with certain other medicines can cause side effects or affect how well clobazam oral suspension or the other medicines work. Do not start or stop other medicines without talking to your healthcare provider. |
|
How should I take****clobazam oral suspension?
|
|
What should I avoid while takingclobazamoral suspension**?** See** "What is the most important information I should know about clobazam oral suspension?"** |
|
What are the possible side effects ofclobazamoral suspension**?** Clobazamoral suspensionmay cause serious side effects, including: See "What is the most important information I should know aboutclobazamoral suspension**?"** The most common side effects of clobazam oral suspension include:
These are not all the possible side effects of clobazam oral suspension. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I storeclobazamoral suspension**?**
Keepclobazamoral suspension****and all medicines out of the reach of children. |
|
General information about the safe and effective use ofclobazamoral suspension**.** Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use clobazam oral suspension for a condition for which it was not prescribed. Do not give clobazam oral suspension to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about clobazam oral suspension that is written for health professionals. |
|
What are the ingredients inclobazamoral suspension**?** **Active ingredient:**clobazam **Inactive ingredients:**artificial mixed berry flavor, citric acid monohydrate, disodium hydrogen phosphate dihydrate, magnesium aluminum silicate, maltitol solution, methylparaben, polysorbate 80, propylene glycol, propylparaben, purified water, simethicone emulsion, sucralose powder, and xanthan gum. Distributed by: Bionpharma Inc., Princeton, NJ 08540 For more information about clobazam oral suspension, callBionpharma Inc. at 1-888-235-2466. |
This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 02/2023 FDA-08
Instructions for Use
**Clobazam (**KLOE-ba-zam)
Oral Suspension, CIV
Read this Instructions for Use before using clobazam oral suspension and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical condition or treatment.
Prepare Clobazam Oral Suspension Dose
You will need the following supplies:See Figure A
- Clobazam oral suspension bottle
- Bottle adapter
- Oral dosing syringe (2 dosing oral syringes are included in the clobazam oral suspension box).
- Use only 1 oral syringe to take your dose of clobazam oral suspension. If you lose or damage the oral syringe, or cannot read the markings, use the other oral syringe.
Figure A
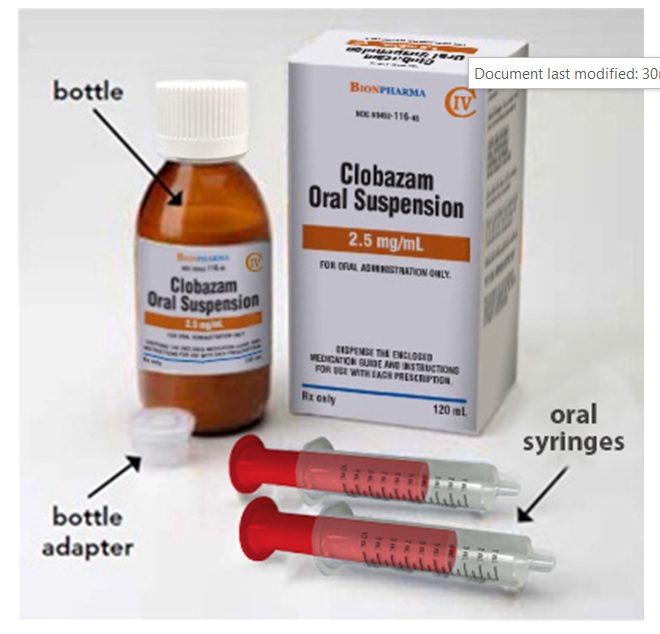
**Step 1.**Remove the clobazam oral suspension bottle, bottle adapter, and 1 oral syringe from the box.
**Step 2.**Shake the bottle well before each use.See****Figure B
Figure B

**Step 3.**Uncap the bottle and firmly insert the bottle adapter into the bottle until the adapter top is even with the bottle top.See Figure C
Figure C
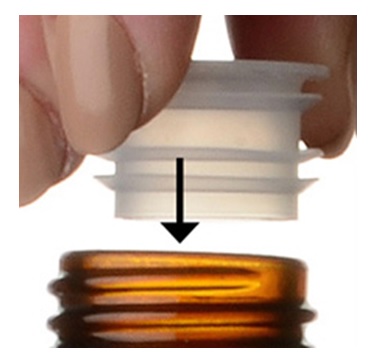
Once the bottle adapter is in place, it should not be removed.
**Step 4.**Check your dose in milliliters (mL) as prescribed by your healthcare provider. Find this number on the oral syringe. Do not take more than the prescribed total dose in 1 day.See Figure D
Figure D
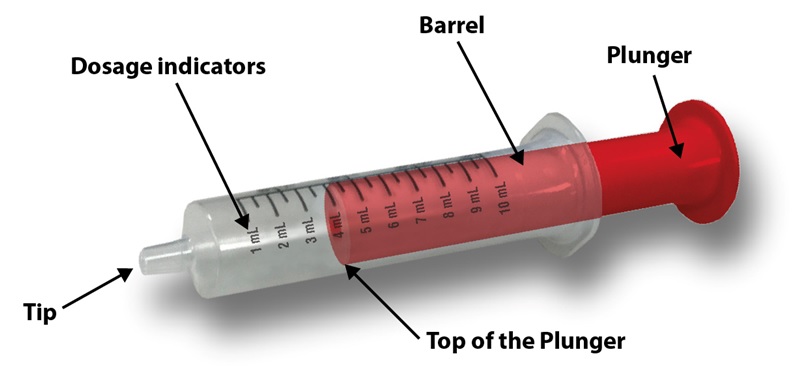
**Step 5.**Push the plunger all the way down and then insert the oral syringe into the upright bottle through the opening in the bottle adapter.See Figure E
Figure E

**Step 6.**With the oral syringe in place, turn the bottle upside down. Pull the plunger to the number of mLs needed (the amount of liquid medicine in Step 4).See Figure F
Figure F

Measure the mLs of medicine using the top of the red plunger.See Figure G
Figure G

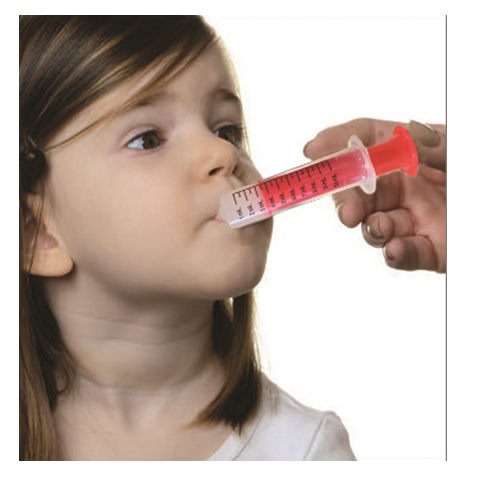
**Step 7.**Remove the oral syringe from the bottle adapter. Slowly squirt clobazam oral suspension directly into the corner of your mouth or your child's mouth until all of the liquid medicine in the oral syringe is given. See Figure H
Figure H
**Step 8.**Cap the bottle tightly with the adapter in place. If the cap does not fit securely, check to see if the adapter is fully inserted.See Figure I
- Store and dispense clobazam oral suspension in its original bottle in an upright position at 68° to 77°F (20° to 25°C).
- Use clobazam oral suspension within 90 days of first opening bottle.
- After 90 days safely throw away any clobazam oral suspension that has not been used.
Figure I
**Step 9.**Wash the oral syringe after each use.
- To clean the oral syringe, take apart by removing the plunger completely. Pull plunger straight out of the barrel.
- The barrel and plunger can be washed with soap and water, rinsed, and allowed to dry.
- Do not wash the oral syringe in the dishwasher.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by:
Bionpharma Inc.
Princeton, NJ 08540
Rev. 02/2023
FDA - 07