Irbesartan and Hydrochlorothiazide
These highlights do not include all the information needed to use IRBESARTAN and HYDROCHLOROTHIAZIDE TABLETS safely and effectively. See full prescribing information for IRBESARTAN and HYDROCHLOROTHIAZIDE TABLETS.IRBESARTAN and HYDROCHLOROTHIAZIDE tablets, for oral useInitial U.S. Approval: 1997
72fa8cd4-bc2a-4a7d-8feb-2d60e077d801
HUMAN PRESCRIPTION DRUG LABEL
Jun 27, 2023
Solco Healthcare US, LLC
DUNS: 828343017
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Irbesartan and Hydrochlorothiazide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
Irbesartan and Hydrochlorothiazide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 300/12.5 mg label
** NDC 43547-331-03 Rx only**
Irbesartan and****Hydrochlorothiazide Tablets USP
300 mg/12.5 mg****30 Tablets
Each film-coated tablet contains: 300 mg of Irbesartan, USP and 12.5 mg of Hydrochlorothiazide, USP.
See package insert for dosing information.
**Store at 20°C - 25°C (68°F - 77°F); excursion permitted to 15°C - 30°C (59°F
- 86°F).** [See USP Controlled Room Temperature]
Manufactured by:
Zhejiang Huahai Pharmaceutical Co., Ltd.
Xunqiao, Linhai, Zhejiang 317024, China
Distributed by:
Solco Healthcare US, LLC
Somerset, NJ 08873, USA
Rev.: 06/2025
200238-03

INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Irbesartan and hydrochlorothiazide tablets are indicated for the treatment of hypertension.
Irbesartan and hydrochlorothiazide tablets may be used in patients whose blood pressure is not adequately controlled on monotherapy.
Irbesartan and hydrochlorothiazide tablets may also be used as initial therapy in patients who are likely to need multiple drugs to achieve their blood pressure goals.
The choice of irbesartan and hydrochlorothiazide tablets as initial therapy for hypertension should be based on an assessment of potential benefits and risks.
Patients with stage 2 (moderate or severe) hypertension are at relatively high risk for cardiovascular events (such as strokes, heart attacks, and heart failure), kidney failure, and vision problems, so prompt treatment is clinically relevant. The decision to use a combination as initial therapy should be individualized and may be shaped by considerations such as the baseline blood pressure, the target goal, and the incremental likelihood of achieving goal with a combination compared with monotherapy.
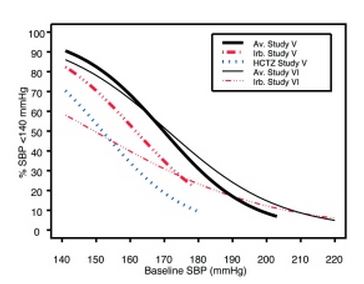
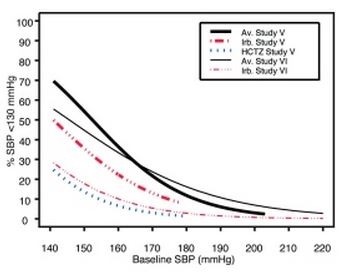
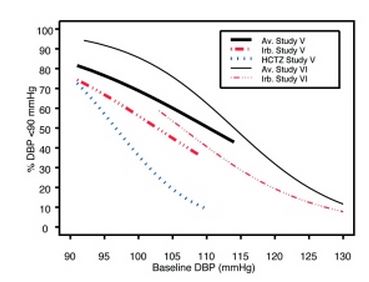
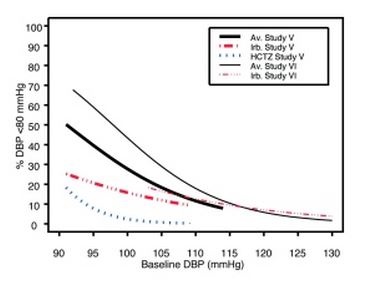
Data from Studies V and VI [see Clinical Studies (14.2)] provide estimates of the probability of reaching a blood pressure goal with irbesartan and hydrochlorothiazide tablets compared to irbesartan or hydrochlorothiazide (HCTZ) monotherapy. The relationship between baseline blood pressure and achievement of a SeSBP <140 or <130 mmHg or SeDBP <90 or <80 mmHg in patients treated with irbesartan and hydrochlorothiazide tablets compared to patients treated with irbesartan or HCTZ monotherapy are shown in Figures 1a through 2b.
|
Figure 1a: Probability of Achieving SBP <140 mmHg in Patients from Initial Therapy Studies V (Week 8) and VI (Week 7) * |
Figure 1b: Probability of Achieving SBP <130 mmHg in Patients from Initial Therapy Studies V (Week 8) and VI (Week 7) * |
|
Figure 2a: Probability of Achieving DBP <90 mmHg in Patients from Initial Therapy Studies V (Week 8) and VI (Week 7) * |
Figure 2b: Probability of Achieving DBP <80 mmHg in Patients from Initial Therapy Studies V (Week 8) and VI (Week 7) * |
The above graphs provide a rough approximation of the likelihood of reaching a targeted blood pressure goal (e.g., Week 8 sitting systolic blood pressure ≤140 mmHg) for the treatment groups. The curve of each treatment group in each study was estimated by logistic regression modeling from all available data of that treatment group. The estimated likelihood at the right tail of each curve is less reliable due to small numbers of subjects with high baseline blood pressures.
For example, a patient with a blood pressure of 180/105 mmHg has about a 25% likelihood of achieving a goal of <140 mmHg (systolic) and 50% likelihood of achieving <90 mmHg (diastolic) on irbesartan alone (and lower still likelihoods on HCTZ alone).
The likelihood of achieving these goals on irbesartan and hydrochlorothiazide tablets rise to about 40% (systolic) or 70% (diastolic).
Irbesartan and hydrochlorothiazide tablets are a combination of irbesartan, an angiotensin II receptor antagonist, and hydrochlorothiazide, a thiazide diuretic, indicated for hypertension:
•
In patients not adequately controlled with monotherapy. (1)
•
As initial therapy in patients likely to need multiple drugs to achieve their blood pressure goals. (1)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
Irbesartan and Hydrochlorothiazide
Irbesartan and hydrochlorothiazide tablets have been evaluated for safety in 1694 patients treated for essential hypertension in 6 clinical trials. In Studies I through IV with irbesartan and hydrochlorothiazide tablets, no adverse events peculiar to this combination drug product have been observed. Adverse events have been limited to those that were reported previously with irbesartan or hydrochlorothiazide (HCTZ). The overall incidence of adverse events was similar with the combination and placebo. In general, treatment with irbesartan and hydrochlorothiazide tablets were well tolerated. For the most part, adverse events have been mild and transient in nature and have not required discontinuation of therapy. In controlled clinical trials, discontinuation of irbesartan and hydrochlorothiazide tablets therapy due to clinical adverse events was required in only 3.6%. This incidence was significantly less (p=0.023) than the 6.8% of patients treated with placebo who discontinued therapy.
In these double-blind controlled clinical trials, the following adverse events reported with irbesartan and hydrochlorothiazide tablets occurred in ≥1% of patients, and more often on the irbesartan and hydrochlorothiazide combination than on placebo, regardless of drug relationship:
|
Irbesartan/HCTZ |
Placebo |
Irbesartan |
HCTZ | |
|---|---|---|---|---|
|
Body as a Whole | ||||
|
Chest Pain |
2 |
1 |
2 |
2 |
|
Fatigue |
6 |
3 |
4 |
3 |
|
Influenza |
3 |
1 |
2 |
2 |
|
Cardiovascular | ||||
|
Edema |
3 |
3 |
2 |
2 |
|
Tachycardia |
1 |
0 |
1 |
1 |
|
Gastrointestinal | ||||
|
Abdominal Pain |
2 |
1 |
2 |
2 |
|
Dyspepsia/heartburn |
2 |
1 |
0 |
2 |
|
Nausea/vomiting |
3 |
0 |
2 |
2 |
|
Immunology | ||||
|
Allergy |
1 |
0 |
1 |
1 |
|
Musculoskeletal | ||||
|
Musculoskeletal Pain |
6 |
5 |
6 |
10 |
|
Nervous System | ||||
|
Dizziness |
8 |
4 |
6 |
5 |
|
Dizziness Orthostatic |
1 |
0 |
1 |
1 |
|
Renal/Genitourinary | ||||
|
Abnormality Urination |
2 |
1 |
1 |
2 |
The following adverse events were also reported at a rate of 1% or greater, but were as, or more, common in the placebo group: headache, sinus abnormality, cough, URI, pharyngitis, diarrhea, rhinitis, urinary tract infection, rash, anxiety/nervousness, and muscle cramp.
Adverse events occurred at about the same rates in men and women, older and younger patients, and black and non-black patients.
Adverse events in Studies V and VI were similar to those described above in Studies I through IV.
Irbesartan
Other adverse events that have been reported with irbesartan, without regard to causality, are listed below:
Body as a Whole: fever, chills, orthostatic effects, facial edema, upper extremity edema
Cardiovascular: flushing, hypertension, cardiac murmur, myocardial infarction, angina pectoris, hypotension, syncope, arrhythmic/conduction disorder, cardiorespiratory arrest, heart failure, hypertensive crisis
Dermatologic: pruritus, dermatitis, ecchymosis, erythema face, urticaria
Endocrine/Metabolic/Electrolyte Imbalances: sexual dysfunction, libido change, gout
Gastrointestinal: diarrhea, constipation, gastroenteritis, flatulence, abdominal distention
Musculoskeletal/Connective Tissue: musculoskeletal trauma, extremity swelling, muscle cramp, arthritis, muscle ache, musculoskeletal chest pain, joint stiffness, bursitis, muscle weakness
Nervous System: anxiety/nervousness, sleep disturbance, numbness, somnolence, vertigo, emotional disturbance, depression, paresthesia, tremor, transient ischemic attack, cerebrovascular accident
Renal/Genitourinary: prostate disorder
Respiratory: cough, upper respiratory infection, epistaxis, tracheobronchitis, congestion, pulmonary congestion, dyspnea, wheezing
Special Senses: vision disturbance, hearing abnormality, ear infection, ear pain, conjunctivitis
Hydrochlorothiazide
Other adverse events that have been reported with hydrochlorothiazide, without regard to causality, are listed below:
Body as a Whole: weakness
Digestive: pancreatitis, jaundice (intrahepatic cholestatic jaundice), sialadenitis, cramping, gastric irritation
Hematologic: aplastic anemia, agranulocytosis, leukopenia, hemolytic anemia, thrombocytopenia
Hypersensitivity: purpura, photosensitivity, urticaria, necrotizing angiitis (vasculitis and cutaneous vasculitis), fever, respiratory distress including pneumonitis and pulmonary edema, anaphylactic reactions
Metabolic: hyperglycemia, glycosuria, hyperuricemia
Musculoskeletal: muscle spasm
Nervous System/Psychiatric: restlessness
Renal: renal failure, renal dysfunction, interstitial nephritis
Skin: erythema multiforme including Stevens-Johnson syndrome, exfoliative dermatitis including toxic epidermal necrolysis
Special Senses: transient blurred vision, xanthopsia
Initial Therapy
In the moderate hypertension Study V (mean SeDBP between 90 and 110 mmHg), the types and incidences of adverse events reported for patients treated with irbesartan and hydrochlorothiazide tablets were similar to the adverse event profile in patients on initial irbesartan or HCTZ monotherapy. There were no reported events of syncope in the irbesartan and hydrochlorothiazide tablets treatment group and there was one reported event in the HCTZ treatment group. The incidences of prespecified adverse events on irbesartan and hydrochlorothiazide tablets, irbesartan, and HCTZ, respectively, were: 0.9%, 0%, and 0% for hypotension; 3.0%, 3.8%, and 1.0% for dizziness; 5.5%, 3.8%, and 4.8% for headache; 1.2%, 0%, and 1.0% for hyperkalemia; and 0.9%, 0%, and 0% for hypokalemia. The rates of discontinuation due to adverse events on irbesartan and hydrochlorothiazide tablets, irbesartan alone, and HCTZ alone were 6.7%, 3.8%, and 4.8%.
In the severe hypertension (SeDBP ≥110 mmHg) Study VI, the overall pattern of adverse events reported through 7 weeks of follow-up was similar in patients treated with irbesartan and hydrochlorothiazide tablets as initial therapy and in patients treated with irbesartan as initial therapy. The incidences of the prespecified adverse events on irbesartan and hydrochlorothiazide tablets and irbesartan, respectively, were: 0% and 0% for syncope; 0.6% and 0% for hypotension; 3.6% and 4.0% for dizziness; 4.3% and 6.6% for headache; 0.2% and 0% for hyperkalemia; and 0.6% and 0.4% for hypokalemia. The rates of discontinuation due to adverse events were 2.1% and 2.2%. [See Clinical Studies (14.2).]
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of irbesartan and hydrochlorothiazide tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Decisions to include these reactions in labeling are typically based on one or more of the following factors: (1) seriousness of the reaction, (2) frequency of reporting, or (3) strength of causal connection to irbesartan and hydrochlorothiazide tablets.
The following have been very rarely reported with irbesartan and hydrochlorothiazide monotherapies: urticaria, jaundice, hepatitis, thrombocytopenia, and impaired renal function including renal failure.
The following have been reported with irbesartan monotherapy: tinnitus, hyperkalemia, angioedema (involving swelling of the face, lips, pharynx, and/or tongue), anaphylactic reaction including anaphylactic shock, increased CPK, anemia, and hypoglycemia in diabetic patients.
The following have been reported with hydrochlorothiazide monotherapy: acute angle-closure glaucoma, acute myopia, and choroidal effusion.
Non-melanoma Skin Cancer
Hydrochlorothiazide is associated with an increased risk of non-melanoma skin cancer. In a study conducted in the Sentinel System, increased risk was predominantly for squamous cell carcinoma (SCC) and in white patients taking large cumulative doses. The increased risk for SCC in the overall population was approximately 1 additional case per 16,000 patients per year, and for white patients taking a cumulative dose of ≥50,000 mg the risk increase was approximately 1 additional SCC case for every 6,700 patients per year.
6.3 Laboratory Abnormalities
In controlled clinical trials, clinically important changes in standard laboratory parameters were rarely associated with administration of irbesartan and hydrochlorothiazide tablets.
Creatinine, Blood Urea Nitrogen: Minor increases in blood urea nitrogen (BUN) or serum creatinine were observed in 2.3% and 1.1%, respectively, of patients with essential hypertension treated with irbesartan and hydrochlorothiazide tablets alone. No patient discontinued taking irbesartan and hydrochlorothiazide tablets due to increased BUN. One patient discontinued taking irbesartan and hydrochlorothiazide tablets due to a minor increase in serum creatinine.
Liver Function Tests: Occasional elevations of liver enzymes and/or serum bilirubin have occurred. In patients with essential hypertension treated with irbesartan and hydrochlorothiazide tablets alone, one patient was discontinued due to elevated liver enzymes.
Serum Electrolytes: [See Warnings and Precautions (5.2, 5.6).]
Most common adverse events (≥ 5% on irbesartan and hydrochlorothiazide tablets and more often than on placebo) are dizziness, fatigue, and musculoskeletal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Solco Healthcare US, LLC at 1-866-257-2597 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DESCRIPTION SECTION
11 DESCRIPTION
Irbesartan and hydrochlorothiazide tablets are a combination of an angiotensin II receptor antagonist (AT1 subtype), irbesartan, and a thiazide diuretic, hydrochlorothiazide (HCTZ).
Irbesartan is a non-peptide compound, chemically described as a 2-butyl-3-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]-1,3-diazaspiro[4.4]non-1-en-4-one. Its empirical formula is C25H28N6O, and its structural formula is:
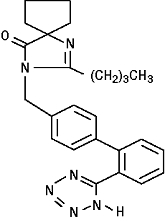
Irbesartan, USP, is a white to off-white crystalline powder with a molecular weight of 428.5. It is a nonpolar compound with a partition coefficient (octanol/water) of 10.1 at pH of 7.4. Irbesartan is slightly soluble in alcohol and methylene chloride and practically insoluble in water.
Hydrochlorothiazide is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2 and its structural formula is:
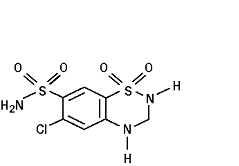
Hydrochlorothiazide, USP, is a white, or practically white, crystalline powder with a molecular weight of 297.7. Hydrochlorothiazide is slightly soluble in water and freely soluble in sodium hydroxide solution.
Irbesartan and hydrochlorothiazide tablets, USP, are available for oral administration in film-coated tablets containing either 150 mg or 300 mg of irbesartan combined with 12.5 mg of hydrochlorothiazide. All dosage strengths contain the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, hypromellose, magnesium stearate, polyvinyl alcohol-part.hydrolyzed, titanium dioxide, talc, macrogol/PEG 3350, lecithin, ferric oxide red, ferric oxide yellow, ferric oxide black.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Irbesartan Monotherapy
The antihypertensive effects of irbesartan were examined in 7 major placebo- controlled, 8 to 12-week trials in patients with baseline diastolic blood pressures of 95 to 110 mmHg. Doses of 1 to 900 mg were included in these trials in order to fully explore the dose-range of irbesartan. These studies allowed a comparison of once or twice-daily regimens at 150 mg/day, comparisons of peak and trough effects, and comparisons of response by gender, age, and race. Two of the 7 placebo-controlled trials identified above and 2 additional placebo-controlled studies examined the antihypertensive effects of irbesartan and hydrochlorothiazide in combination.
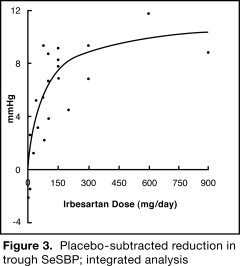
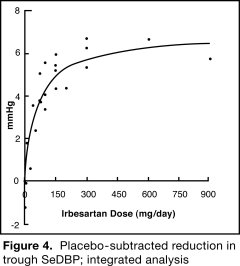
The 7 studies of irbesartan monotherapy included a total of 1915 patients randomized to irbesartan (1 to 900 mg) and 611 patients randomized to placebo. Once-daily doses of 150 to 300 mg provided statistically and clinically significant decreases in systolic and diastolic blood pressure with trough (24-hour post dose) effects after 6 to 12 weeks of treatment compared to placebo, of about 8 to 10/5 to 6 mmHg and 8 to 12/5 to 8 mmHg, respectively. No further increase in effect was seen at dosages greater than 300 mg. The dose-response relationships for effects on systolic and diastolic pressure are shown in Figures 3 and 4.
|
|
|
Once-daily administration of therapeutic doses of irbesartan gave peak effects at around 3 to 6 hours and, in one continuous ambulatory blood pressure monitoring study, again around 14 hours. This was seen with both once-daily and twice-daily dosing. Trough-to-peak ratios for systolic and diastolic response were generally between 60% and 70%. In a continuous ambulatory blood pressure monitoring study, once-daily dosing with 150 mg gave trough and mean 24-hour responses similar to those observed in patients receiving twice-daily dosing at the same total daily dose.
Analysis of age, gender, and race subgroups of patients showed that men and women, and patients over and under 65 years of age, had generally similar responses. Irbesartan was effective in reducing blood pressure regardless of race, although the effect was somewhat less in blacks (usually a low-renin population). Black patients typically show an improved response with the addition of a low dose diuretic (e.g., 12.5 mg hydrochlorothiazide).
The effect of irbesartan is apparent after the first dose and is close to the full observed effect at 2 weeks. At the end of the 8-week exposure, about 2/3 of the antihypertensive effect was still present 1 week after the last dose. Rebound hypertension was not observed. There was essentially no change in average heart rate in irbesartan-treated patients in controlled trials.
14.2 Irbesartan and Hydrochlorothiazide
The antihypertensive effects of irbesartan and hydrochlorothiazide tablets were examined in 4 placebo-controlled studies in patients with mild-moderate hypertension (mean seated diastolic blood pressure [SeDBP] between 90 and 110 mmHg), one study in patients with moderate hypertension (mean seated systolic blood pressure [SeSBP] 160 to 179 mmHg or SeDBP 100 to 109 mmHg), and one study in patients with severe hypertension (mean SeDBP ≥110 mmHg) of 8 to 12 weeks. These trials included 3149 patients randomized to fixed doses of irbesartan (37.5 to 300 mg) and concomitant hydrochlorothiazide (6.25 to 25 mg).
Study I was a factorial study that compared all combinations of irbesartan (37.5 mg, 100 mg, and 300 mg or placebo) and hydrochlorothiazide (6.25 mg, 12.5 mg, and 25 mg or placebo).
Study II compared the irbesartan and hydrochlorothiazide combinations of 75/12.5 mg and 150/12.5 mg to their individual components and placebo.
Study III investigated the ambulatory blood pressure responses to irbesartan and hydrochlorothiazide (75/12.5 mg and 150/12.5 mg) and placebo after 8 weeks of dosing.
Study IV investigated the effects of the addition of irbesartan (75 or 150 mg) in patients not controlled (SeDBP 93-120 mmHg) on hydrochlorothiazide (25 mg) alone. In Studies I–III, the addition of irbesartan 150 to 300 mg to hydrochlorothiazide doses of 6.25, 12.5, or 25 mg produced further dose- related reductions in blood pressure at trough of 8 to 10 mmHg/3 to 6 mmHg, similar to those achieved with the same monotherapy dose of irbesartan. The addition of hydrochlorothiazide to irbesartan produced further dose-related reductions in blood pressure at trough (24 hours post dose) of 5 to 6/2 to 3 mmHg (12.5 mg) and 7 to 11/4 to 5 mmHg (25 mg), also similar to effects achieved with hydrochlorothiazide alone. Once-daily dosing with 150 mg irbesartan and 12.5 mg hydrochlorothiazide, 300 mg irbesartan and 12.5 mg hydrochlorothiazide, or 300 mg irbesartan and 25 mg hydrochlorothiazide produced mean placebo-adjusted blood pressure reductions at trough (24 hours post dosing) of about 13 to 15/7 to 9 mmHg, 14/9 to 12 mmHg, and 19 to 21/11 to 12 mmHg, respectively. Peak effects occurred at 3 to 6 hours, with the trough-to-peak ratios >65%.
In Study IV, the addition of irbesartan (75-150 mg) gave an additive effect (systolic/diastolic) at trough (24 hours post dosing) of 11/7 mmHg.
Initial Therapy
Studies V and VI had no placebo group, so effects described below are not all attributable to irbesartan or HCTZ.
Study V was conducted in patients with a mean baseline blood pressure of 162/98 mmHg and compared the change from baseline in SeSBP at 8 weeks between the combination group (irbesartan and HCTZ 150/12.5 mg), to irbesartan (150 mg) and to HCTZ (12.5 mg). These initial study regimens were increased at 2 weeks to irbesartan and hydrochlorothiazide tablets 300/25 mg, irbesartan 300 mg, or to HCTZ 25 mg, respectively.
Mean reductions from baseline for SeDBP and SeSBP at trough were 14.6 mmHg and 27.1 mmHg for patients treated with irbesartan and hydrochlorothiazide tablets, 11.6 mmHg and 22.1 mmHg for patients treated with irbesartan, and 7.3 mmHg and 15.7 mmHg for patients treated with HCTZ at 8 weeks, respectively. For patients treated with irbesartan and hydrochlorothiazide tablets, the mean change from baseline in SeDBP was 3.0 mmHg lower (p=0.0013) and the mean change from baseline in SeSBP was 5.0 mmHg lower (p=0.0016) compared to patients treated with irbesartan, and 7.4 mmHg lower (p<0.0001) and 11.3 mmHg lower (p<0.0001) compared to patients treated with HCTZ, respectively. Withdrawal rates were 3.8% on irbesartan, 4.8% on HCTZ, and 6.7% on irbesartan and hydrochlorothiazide tablets.
Study VI was conducted in patients with a mean baseline blood pressure of 172/113 mmHg and compared trough SeDBP at 5 weeks between the combination group (irbesartan and HCTZ 150/12.5 mg) and irbesartan (150 mg). These initial study regimens were increased at 1 week to irbesartan and hydrochlorothiazide tablets 300/25 mg or to irbesartan 300 mg, respectively.
At 5 weeks, mean reductions from baseline for SeDBP and SeSBP at trough were 24.0 mmHg and 30.8 mmHg for patients treated with irbesartan and hydrochlorothiazide tablets and 19.3 mmHg and 21.1 mmHg for patients treated with irbesartan, respectively. The mean SeDBP was 4.7 mmHg lower (p<0.0001) and the mean SeSBP was 9.7 mmHg lower (p<0.0001) in the group treated with irbesartan and hydrochlorothiazide tablets than in the group treated with irbesartan. Patients treated with irbesartan and hydrochlorothiazide tablets achieved more rapid blood pressure control with significantly lower SeDBP and SeSBP and greater blood pressure control at every assessment (Week 1, Week 3, Week 5, and Week 7). Maximum effects were seen at Week 7.
Withdrawal rates were 2.2% on irbesartan and 2.1% on irbesartan and hydrochlorothiazide tablets.
In Studies I-VI, there was no difference in response for men and women or in patients over or under 65 years of age. Black patients had a larger response to hydrochlorothiazide than non-black patients and a smaller response to irbesartan. The overall response to the combination was similar for black and non-black patients.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Irbesartan and Hydrochlorothiazide film-coated tablets have markings on both sides and are available in the strengths and packages listed in the following table:
|
Tablet Strength |
Film-Coated Tablet |
Tablet Markings |
Package Size |
NDC Code |
|
150 mg and 12.5 mg |
Pink, |
Debossed '325' on one |
Bottles of 30 |
43547-330-03 |
|
Bottles of 90 |
43547-330-09 | |||
|
Bottles of 500 |
43547-330-50 | |||
|
300 mg and 12.5 mg |
Pink, |
Debossed '327' on one |
Bottles of 30 |
43547-331-03 |
|
Bottles of 90 |
43547-331-09 | |||
|
Bottles of 500 |
43547-331-50 |
16.2 Storage
Store at 20°C-25°C (68°F-77°F); excursions permitted to 15°C-30°C (59°F-86°F) [see USP Controlled Room Temperature].