TOSSOYA Rainforest SecretGuard Antibacterial Quick-Cleanse Wipes
43116-045
3d53463d-8d8e-9030-e063-6294a90ab454
HUMAN OTC DRUG LABEL
Sep 1, 2025
Shenzhen Shierjie Biological Engineering Co., LTD
DUNS: 547610261
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
TOSSOYA Rainforest SecretGuard Antibacterial Quick-Cleanse Wipes
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
TOSSOYA Rainforest SecretGuard Antibacterial Quick-Cleanse Wipes
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
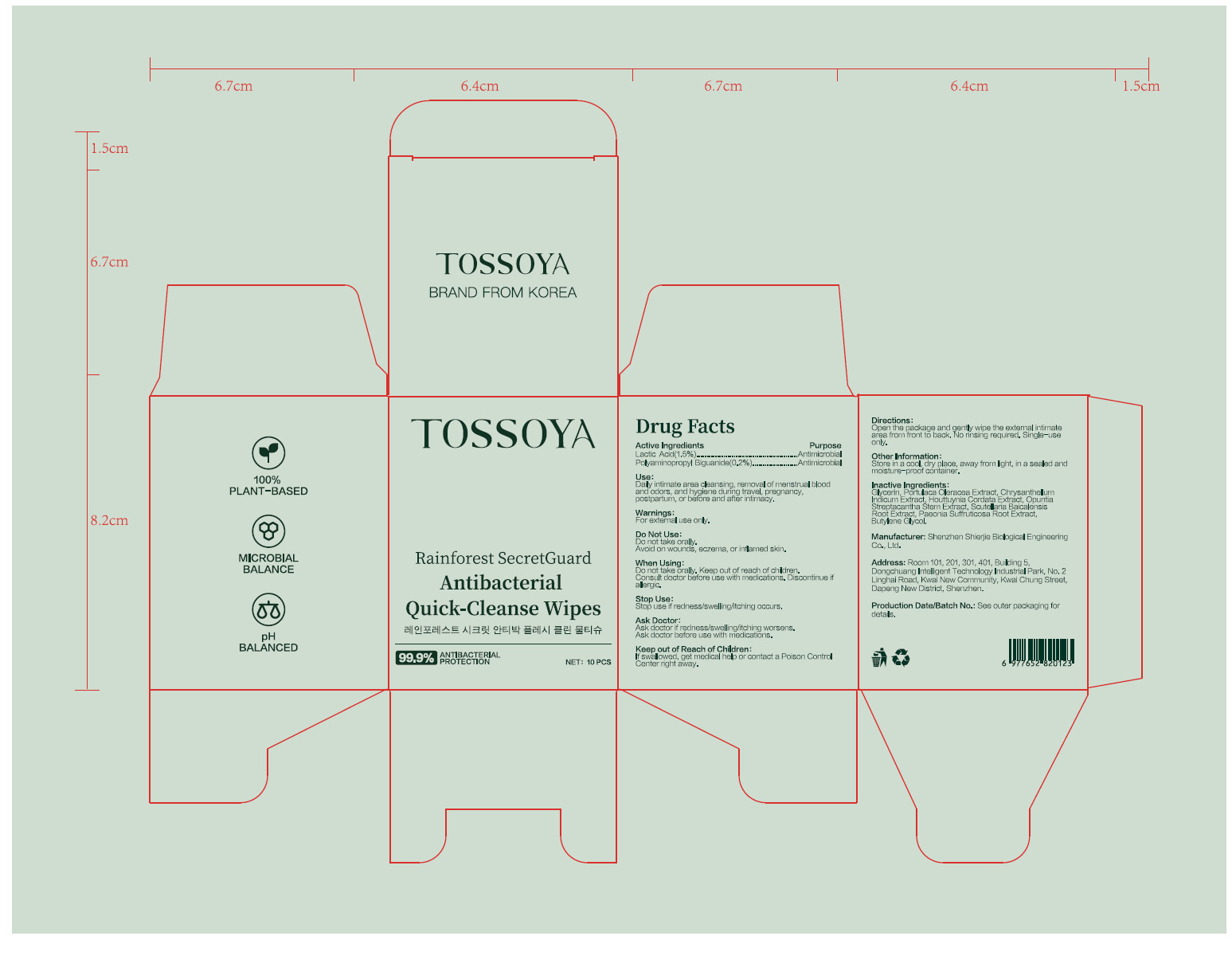
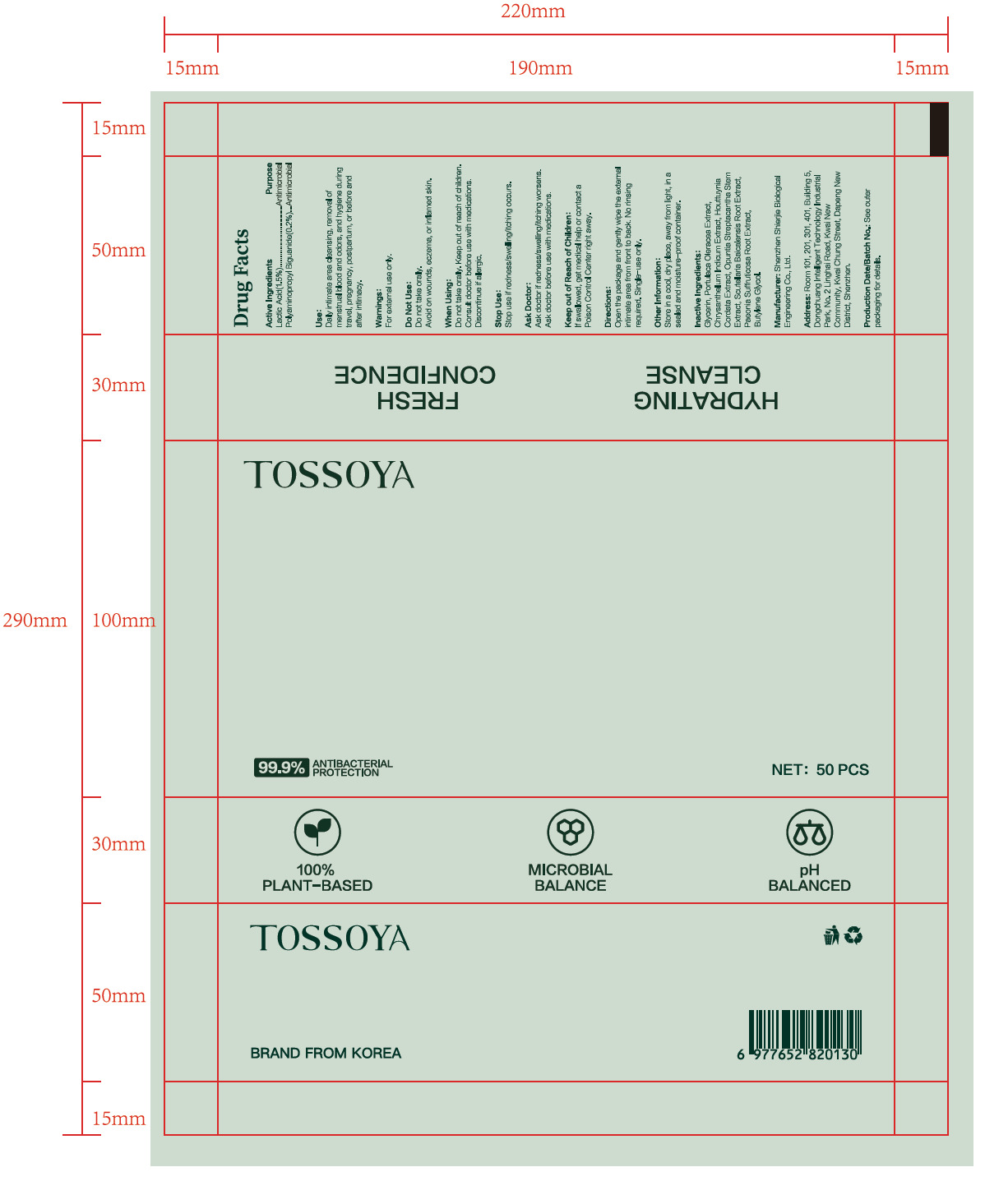
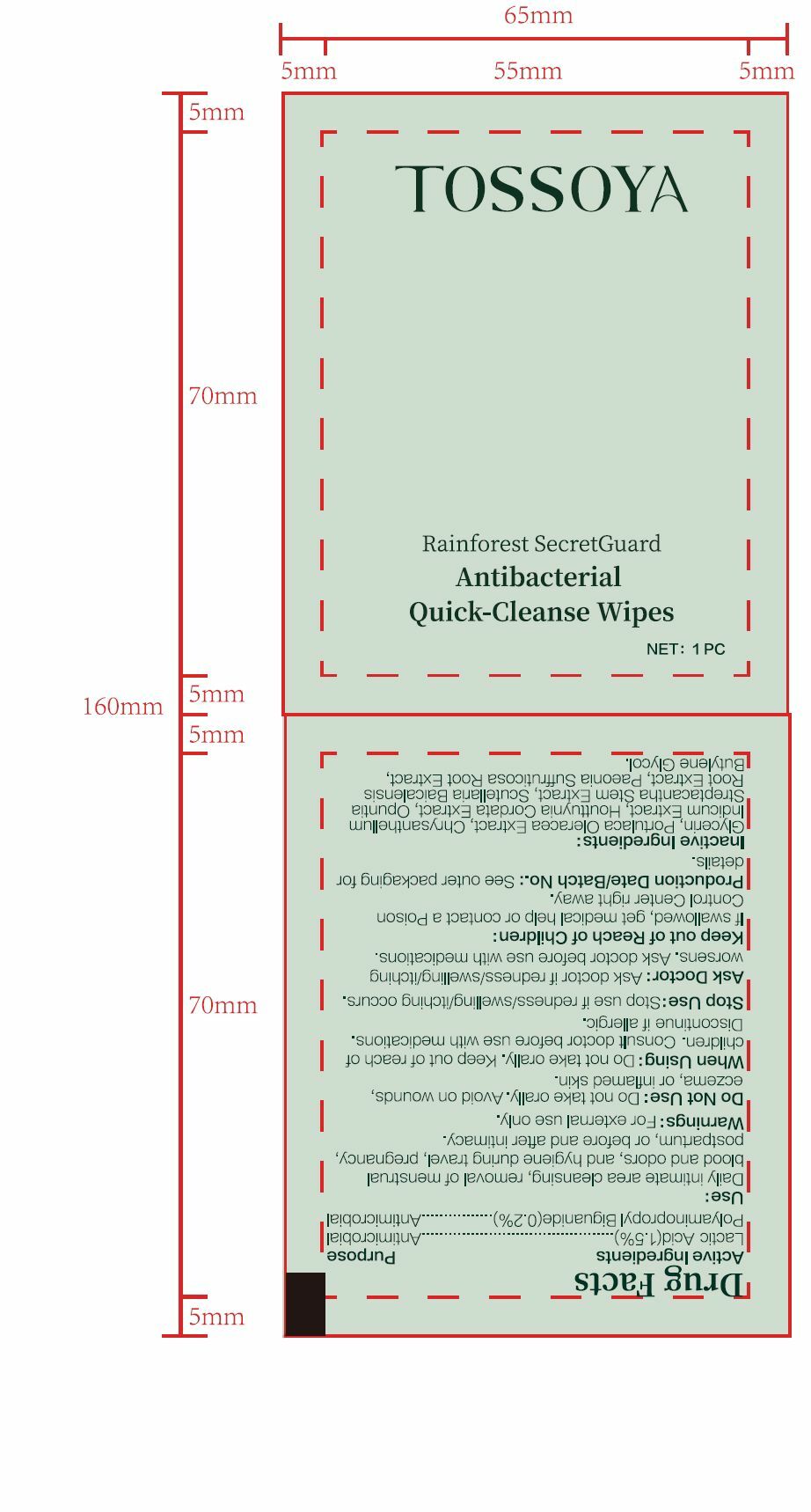
INDICATIONS & USAGE SECTION
Use
Daily intimate area cleansing, removal of menstrual blood and odors, and
hygiene during travel, pregnancy,
postpartum, or before and after intimacy.
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient
Lactic Acid 1.5%
Polyaminopropyl Biguanide 0.2%
STORAGE AND HANDLING SECTION
Other information
Store in a cool, dry place, away from light, in a sealed and
moisture-proof container.
OTC - PURPOSE SECTION
Purpose
Antimicrobial
WARNINGS SECTION
Warnings
For external use only.
OTC - DO NOT USE SECTION
Do not use
Do not take orally.
Avoid on wounds, eczema, or inflamed skin.
OTC - WHEN USING SECTION
When Using
Do not take orally. Keep out of reach of children.
Consult doctor before use with medications. Discontinue if allergic.
OTC - STOP USE SECTION
Stop Use
Stop use if redness/swelling/itching occurS.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep Oot Of Reach Of Children
If swallowed, get medical help or contact a Poison Control
Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
Openthe package and genty wipe the exteral itimate
area from front to back. No rinsing required. Single-use
only.
INACTIVE INGREDIENT SECTION
Inactive ingredients
Glycerin, Portulaca Oleracea Extract, Chrysanthellum Indicum Extract,
Houttuynia Cordata Extract, Opuntia
Streptacantha Stem Extract, Scutellaria Baicalensis Root Extract, Paeonia
Suffruticosa Root Bark Extract, Bifida
Ferment Filtrate, Butylene Glycol.
